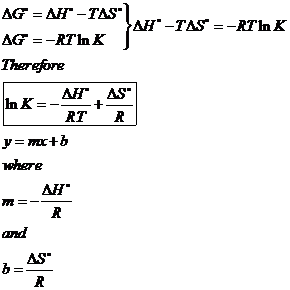How To Calculate Standard Free Energy Change
1725 d g n f e c e l l.
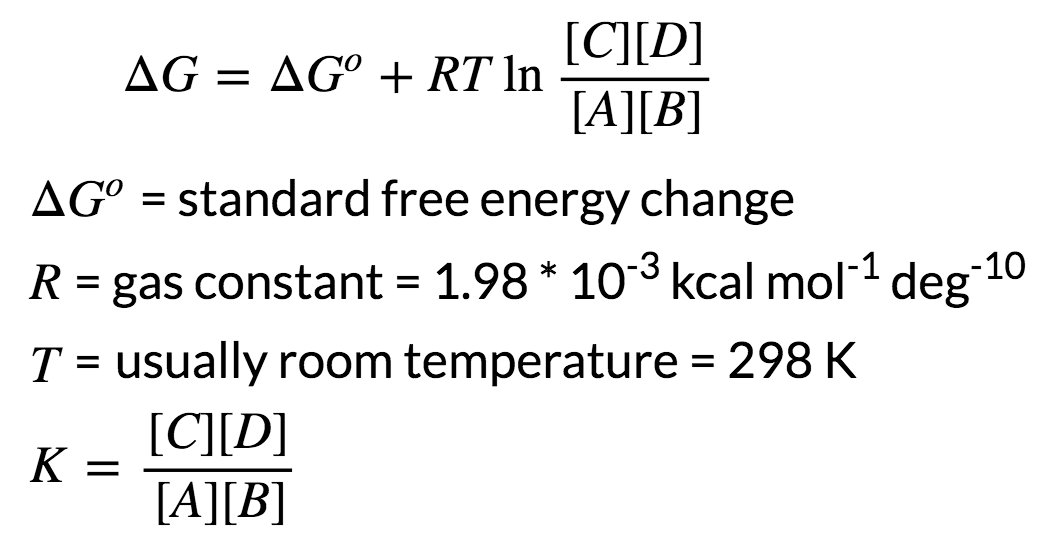
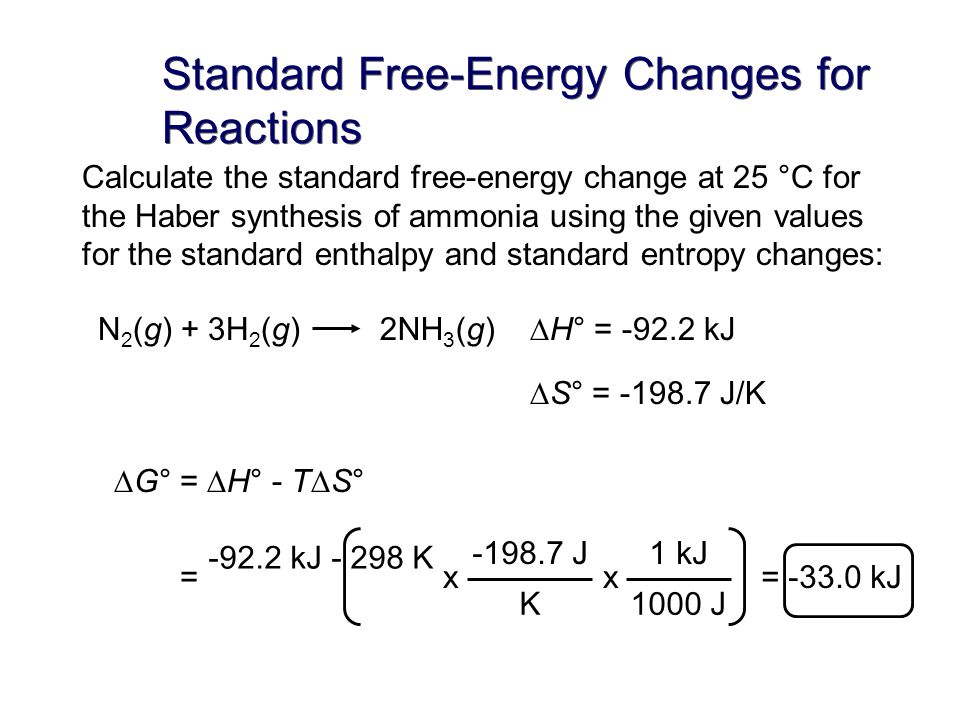
How to calculate standard free energy change. Delta g zero is the standard change in free energy or the change in free energy under standard conditions. Using cell potentials to determine standard state free energy changes. R universal gas constant.
Values for the equilibrium constant for electrochemical cell reactions are sometimes very large. Note that the reaction as written is unfavored. Finally calculate dg0reaction in kj mol 1 at standard temperature t 29815 k and pressure by substituting these values for dh0reaction t reaction in k and ds0reaction into the equation for the change in gibbs free energy of the chemical system as shown below.
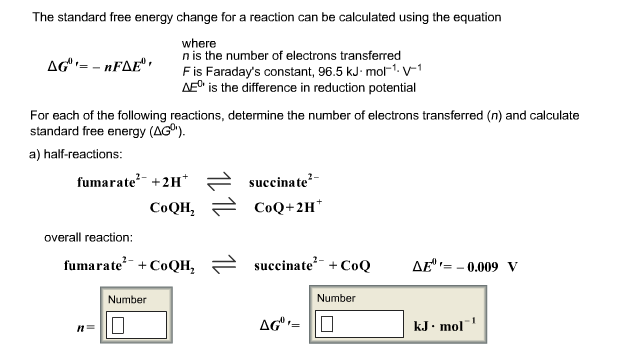
Standard free energy change and equilibrium constant calculator dg o standard free energy change. Dg change in gibbs energy of a reaction or a process indicates whether or not that the reaction occurs spontaniously. N 2 moles of electrons.
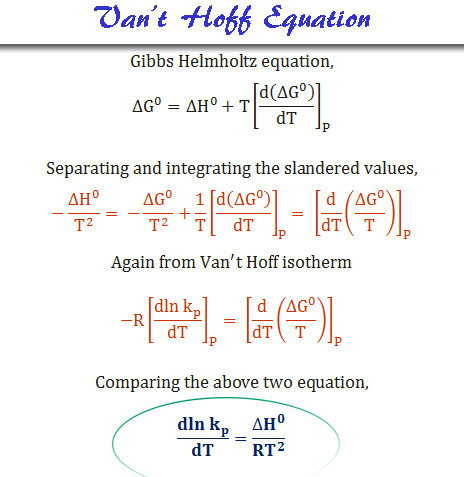
2 ag aq al s 2 ag s cu 2 aq e ocell 0281 v. The standard free energy change of the reaction can be determined by adding the two free energies of reaction. The above equation is one of the most widely used equation in thermodynamics.
In this equation r 8314 j mol 1 k 1 or 0008314 kj mol 1 k 1. The relationship between the free energy of reaction at any moment in time g and the standard state free energy of reaction g o is described by the following equation. T is the temperature on the kelvin scale.
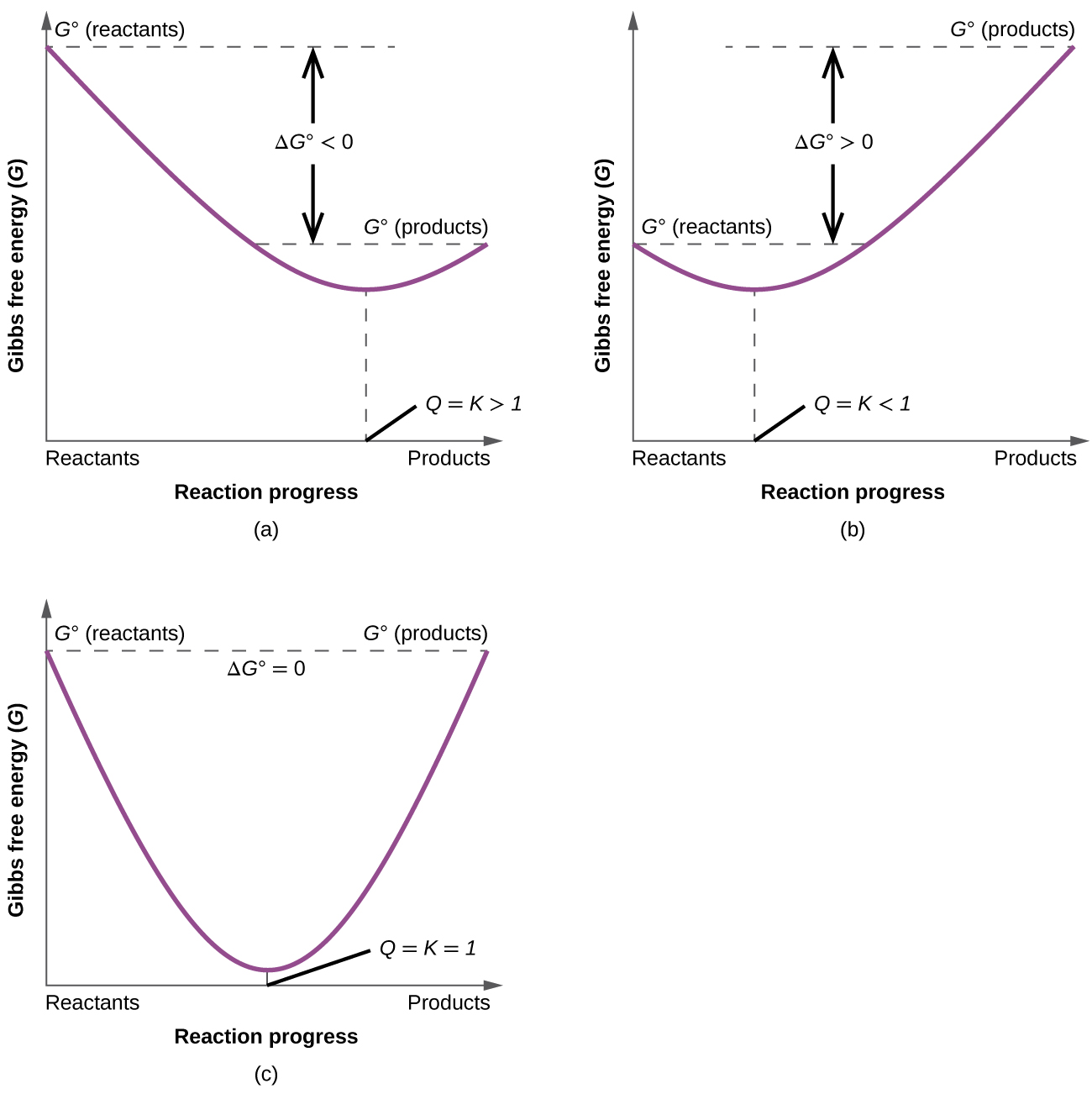
So we have another very important equation to think about. The change in free energy d g is also a measure of the maximum amount of work that can be performed during a chemical process d g w m a x. Consequently there must be a relationship between the potential of an electrochemical cell and d g.
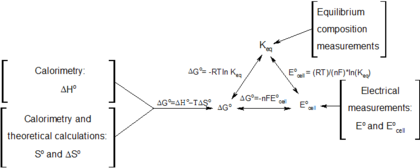
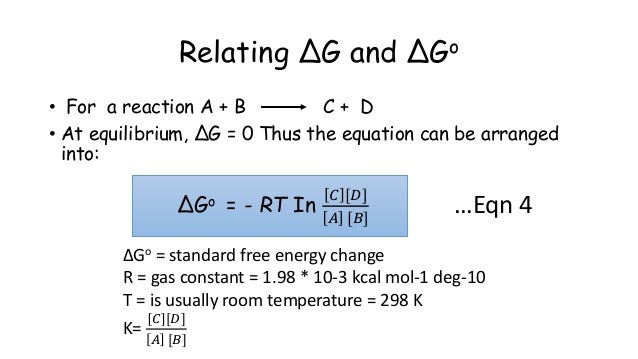
If we know the equilibrium constant k eq for a chemical change or if we can determine the equilibrium constant we can calculate the standard state free energy change g o for the reaction using the equation. Its free energy change is positive. Under standard conditions has dg0 73 kcalmole 31 kjmole.
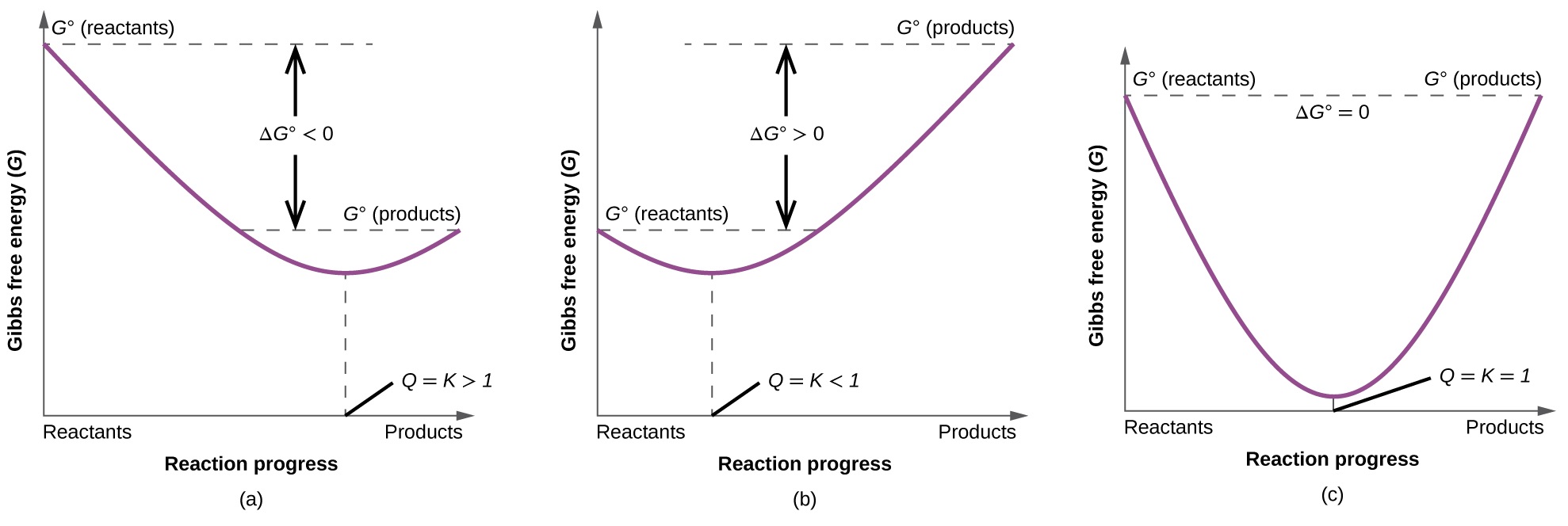
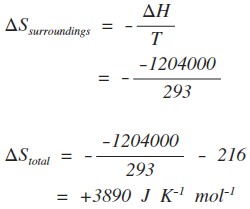
When dg 0 the reaction or a process is at equilibrium. Dgrxn sdg fproductssdg freactants d g rxn s d g f products s d g f reactants can be used to determine standard free energy change of a reaction. This relationship is as follows.
So we solve for delta g zero. Determining the standard state free energy change from e ocell. K eq equilibrium constant.
Substitute into the above equation and solve for k. K eq is the equilibrium constant at the temperature t. R is the gas constant t is the temperature in kelvin and k is our equilibrium constant.
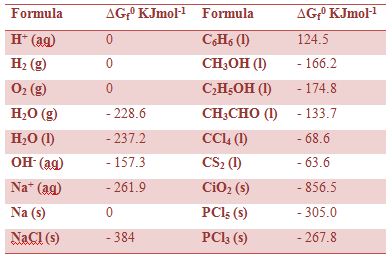
Combine the standard enthalpy of formation and the standard entropy of a substance to get its standard free energy of formation.
Https Www Studocu Com En Gb Document University Of East Anglia Further Chemistry Lecture Notes 05 02 18 Energy Changes In Chemical Reactions 3 1570600 View
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcs4pqwasztj8vdgiebgeyehl8z8oofviuzhglutttyjwtb6dlby Usqp Cau
encrypted-tbn0.gstatic.com

Practice Set 1 1 Practice Set 1 Free Energy Changes And Equilibrium Constants 1 Calculate The Standard Free Energy Changes Of The Following Course Hero
www.coursehero.com
Http Anokahennepin Schoolwires Net Cms Lib08 Mn01909485 Centricity Domain 4810 Chem 202andib 20chem Review Ws Energetics 20part 202 20entropy 20answer Key Pdf
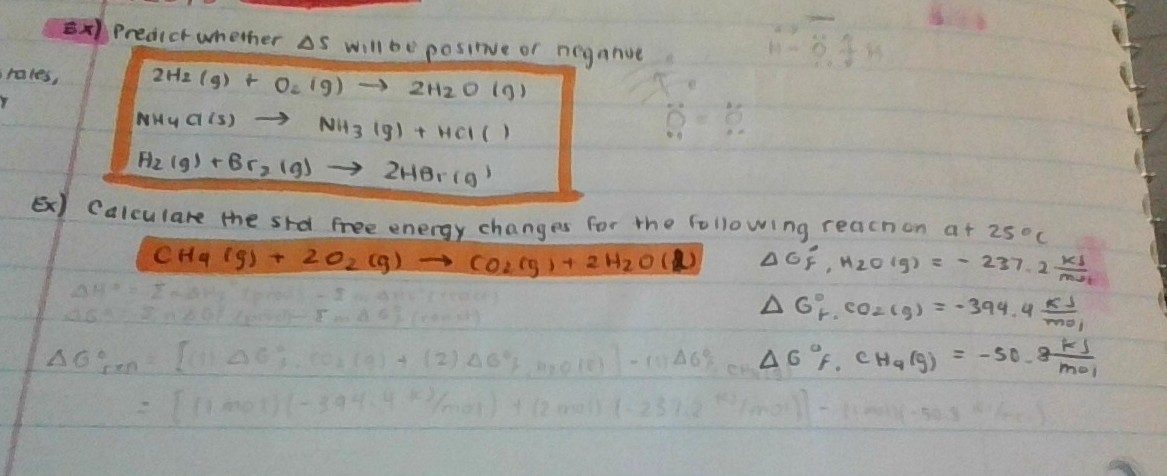
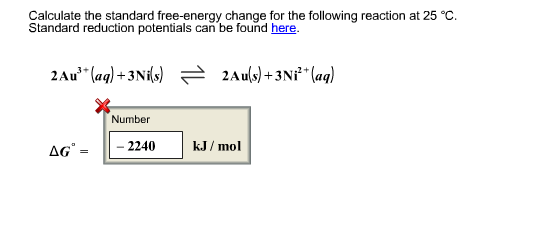
Calculate The Standard Free Energy Change For The Below Reaction At 25 Degrees Celsius Socratic
socratic.org
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcrjzer7vt1q66b7tppiliniux7zvndiv2alafk8pidco0mdbmaj Usqp Cau
encrypted-tbn0.gstatic.com
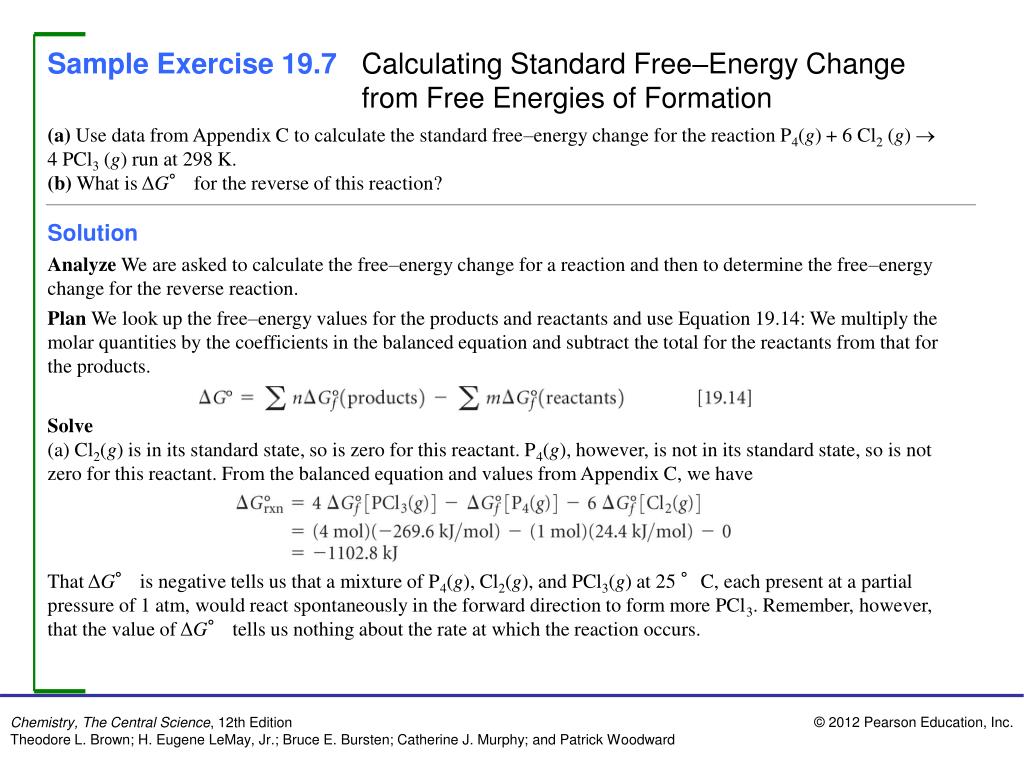
Ppt Sample Exercise 19 1 Identifying Spontaneous Processes Powerpoint Presentation Id 5072617
www.slideserve.com





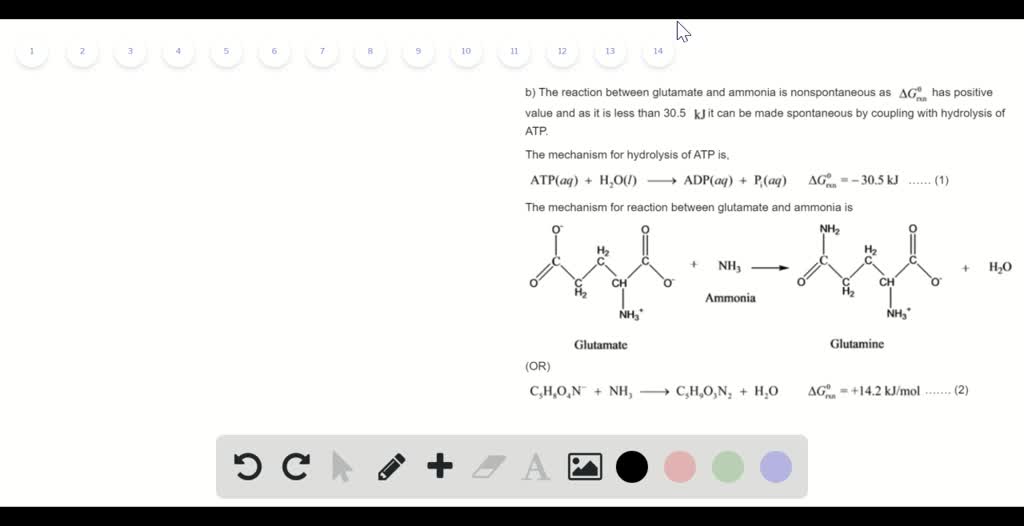
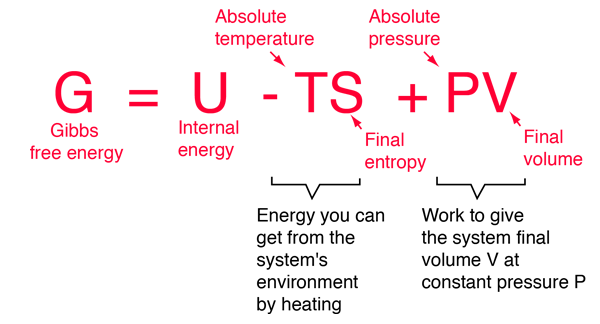


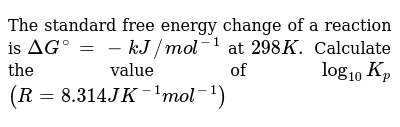
.PNG)



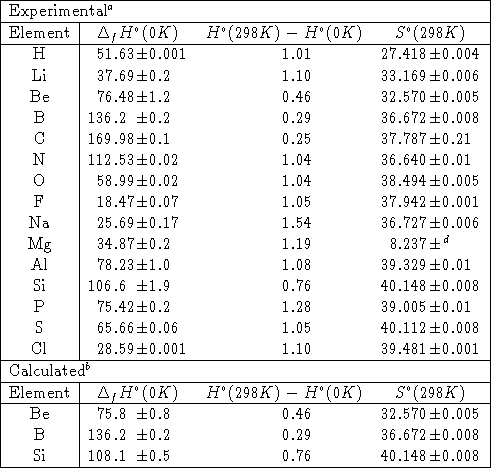





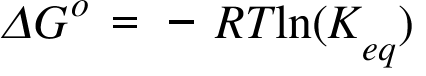







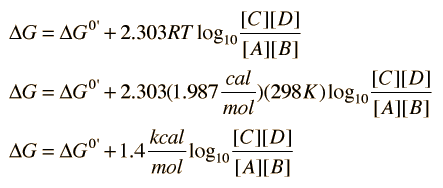

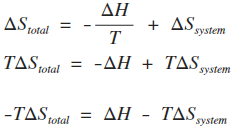


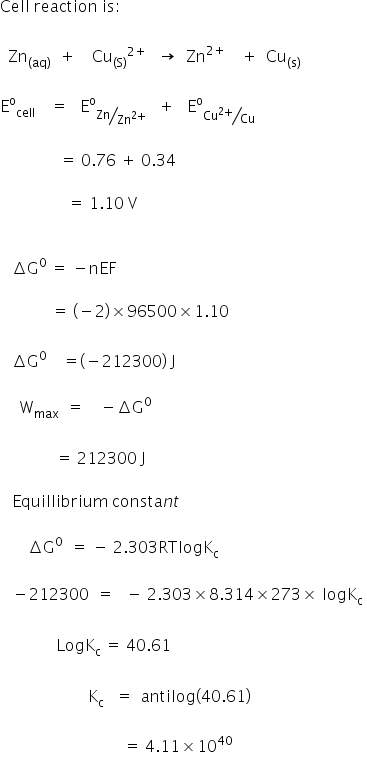





.jpg)