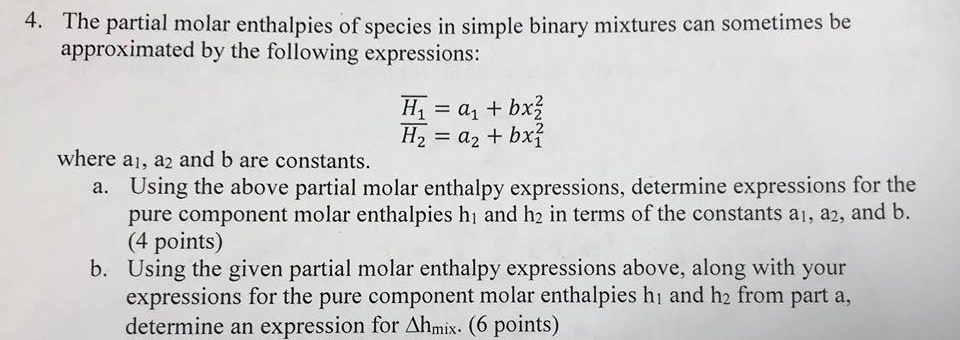
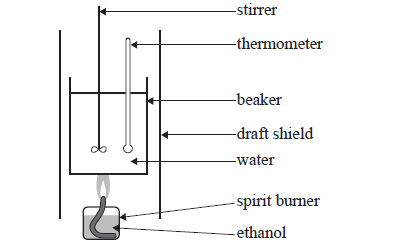
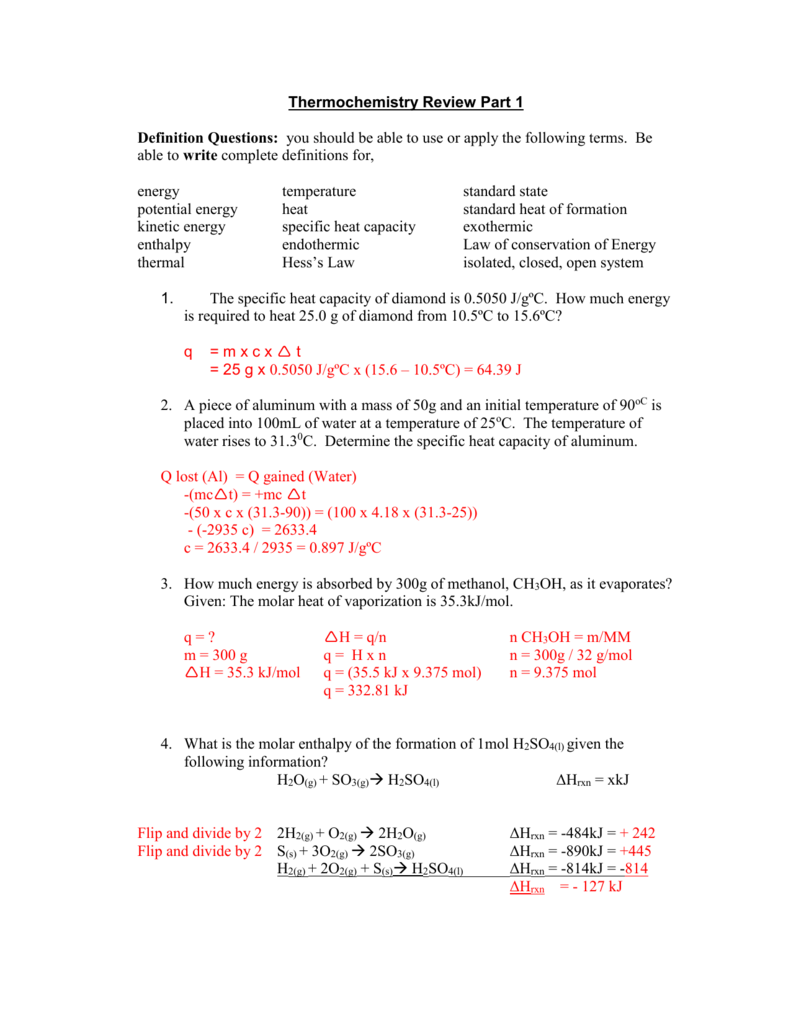
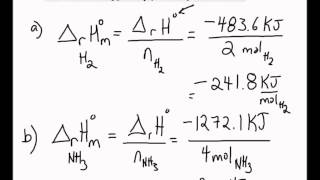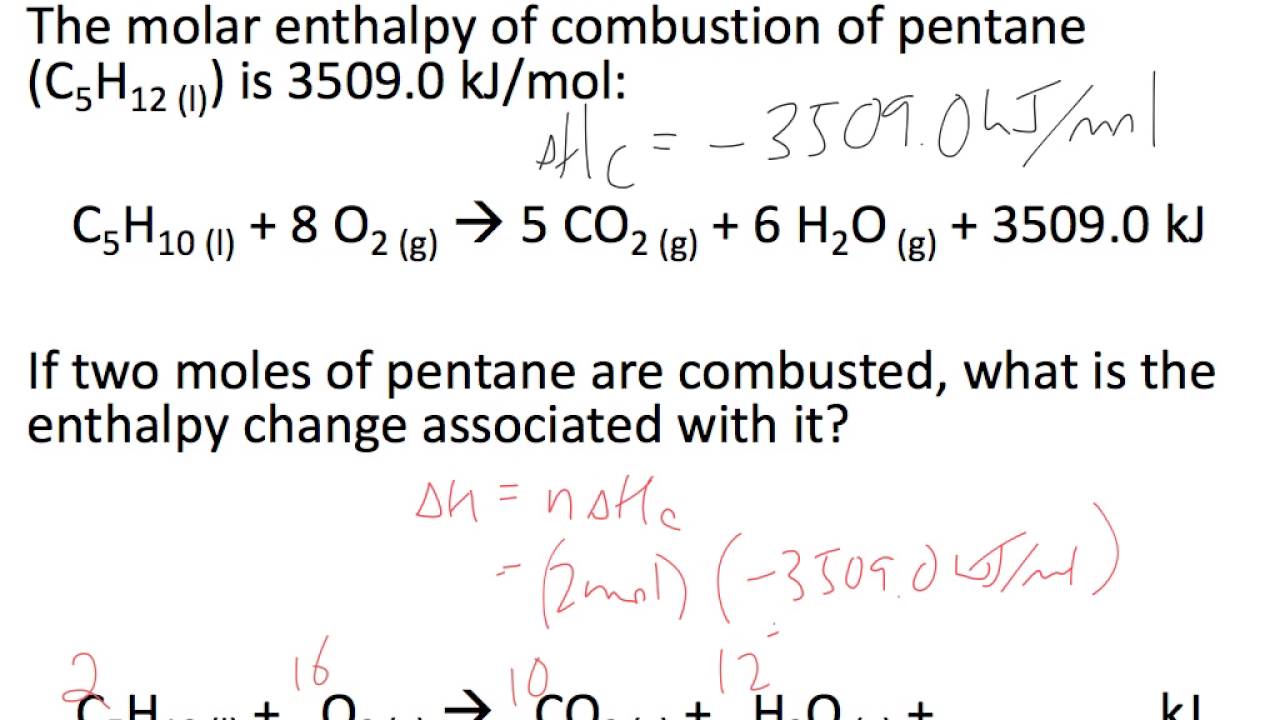How To Determine Molar Enthalpy
A positive value indicates an increase in entropy while a negative value denotes a decrease in the entropy of a system.

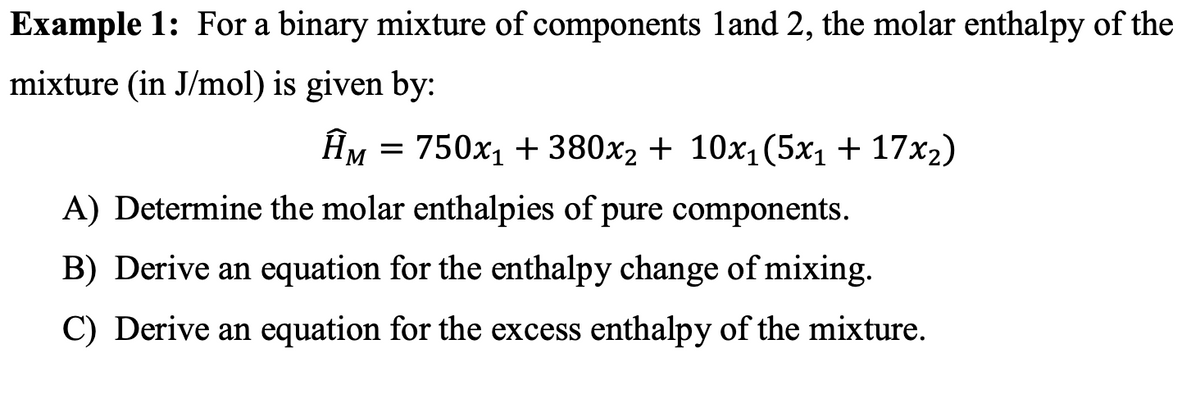
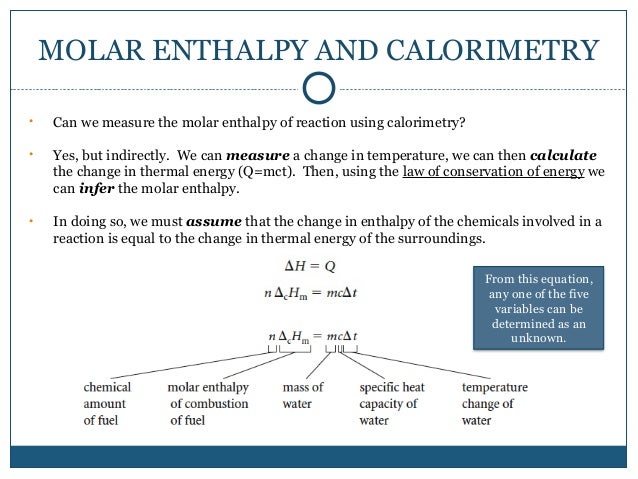
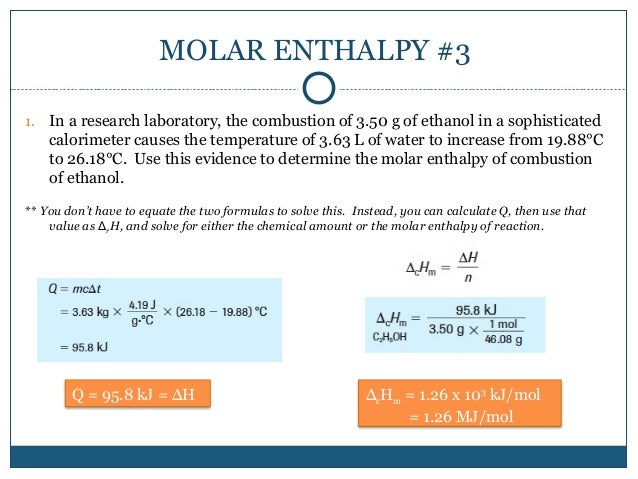
How to determine molar enthalpy. Updated december 03 2019. Finally substitute the values of q t 1 and t 2 in the heat capacity formula. Molar enthalpy d hn.
It really is that simple. H total 6 394 3 286 3267. Usual units of standard molar entropy are joules per mole kelvin jmolk.
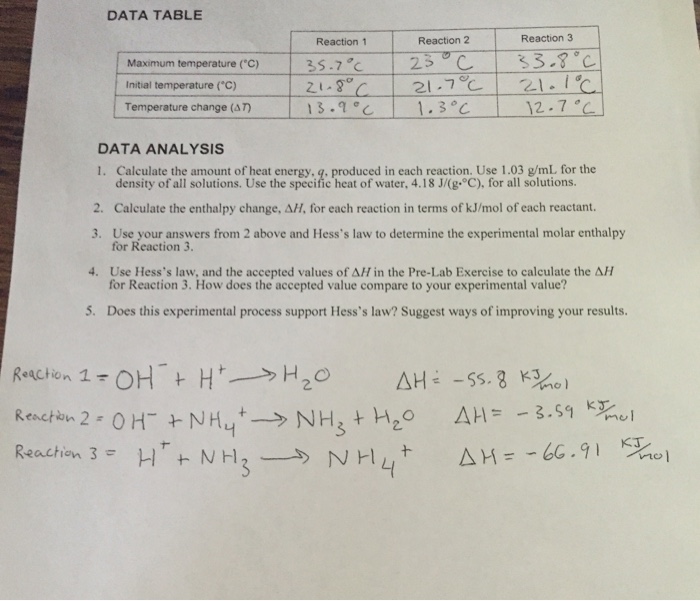
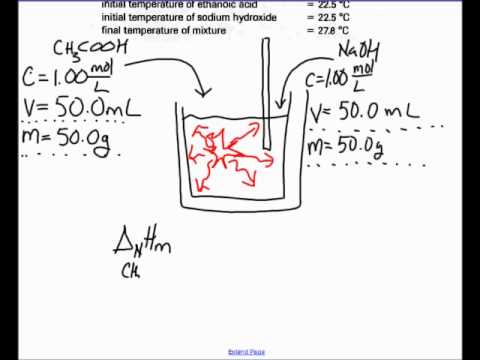
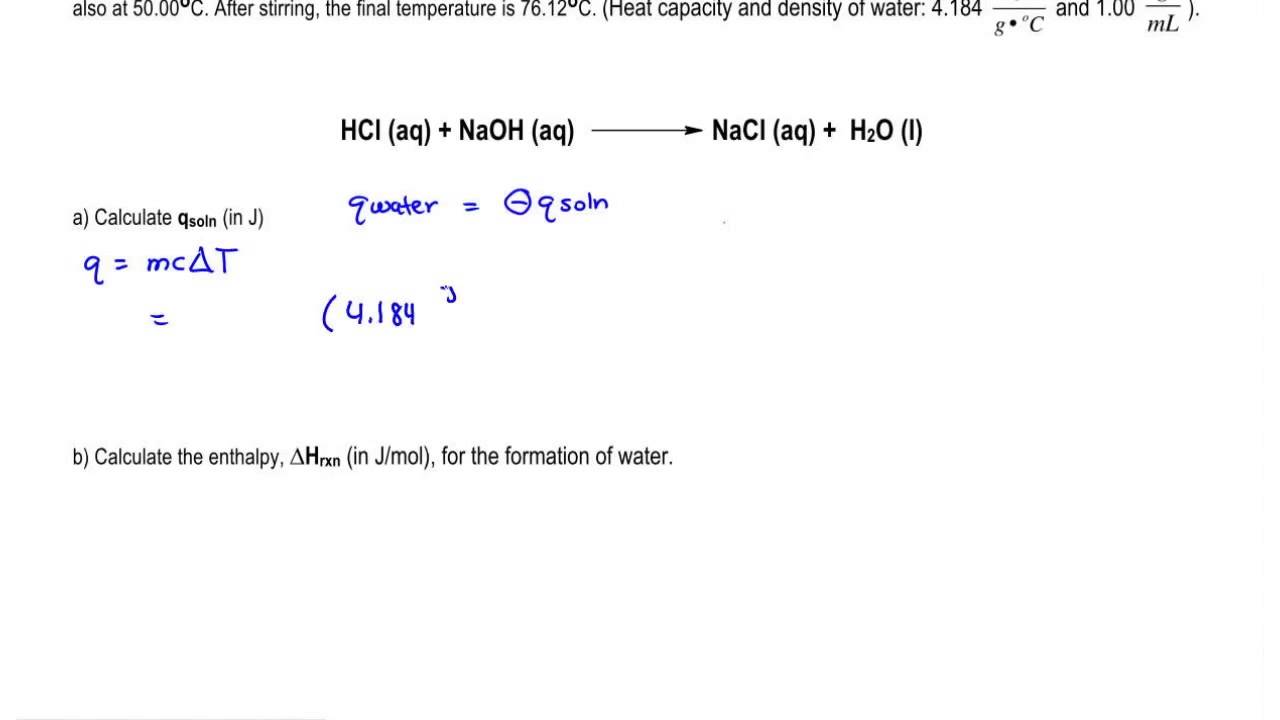
Multiply the amount of heat energy in calories by 4184 to get the amount in joules 1 cal 4184 joules. 10 mol l 1 hcl aq at the same temperature is added 100 ml at a time. In molar heat of neutralization problems n cv where.
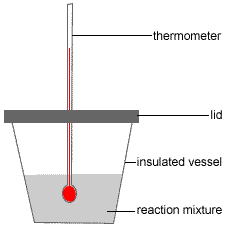
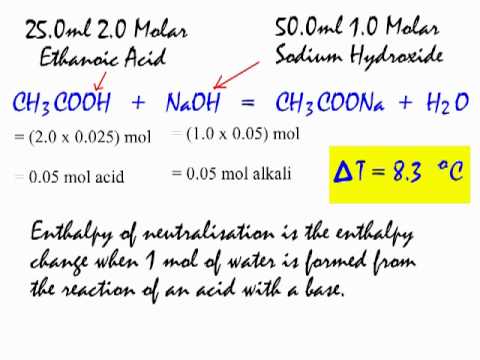
Standard molar entropy is defined as the entropy or degree of randomness of one mole of a sample under standard state conditions. Calculate the reaction enthalpy for the following reaction. The temperature of the naoh aq is recorded.
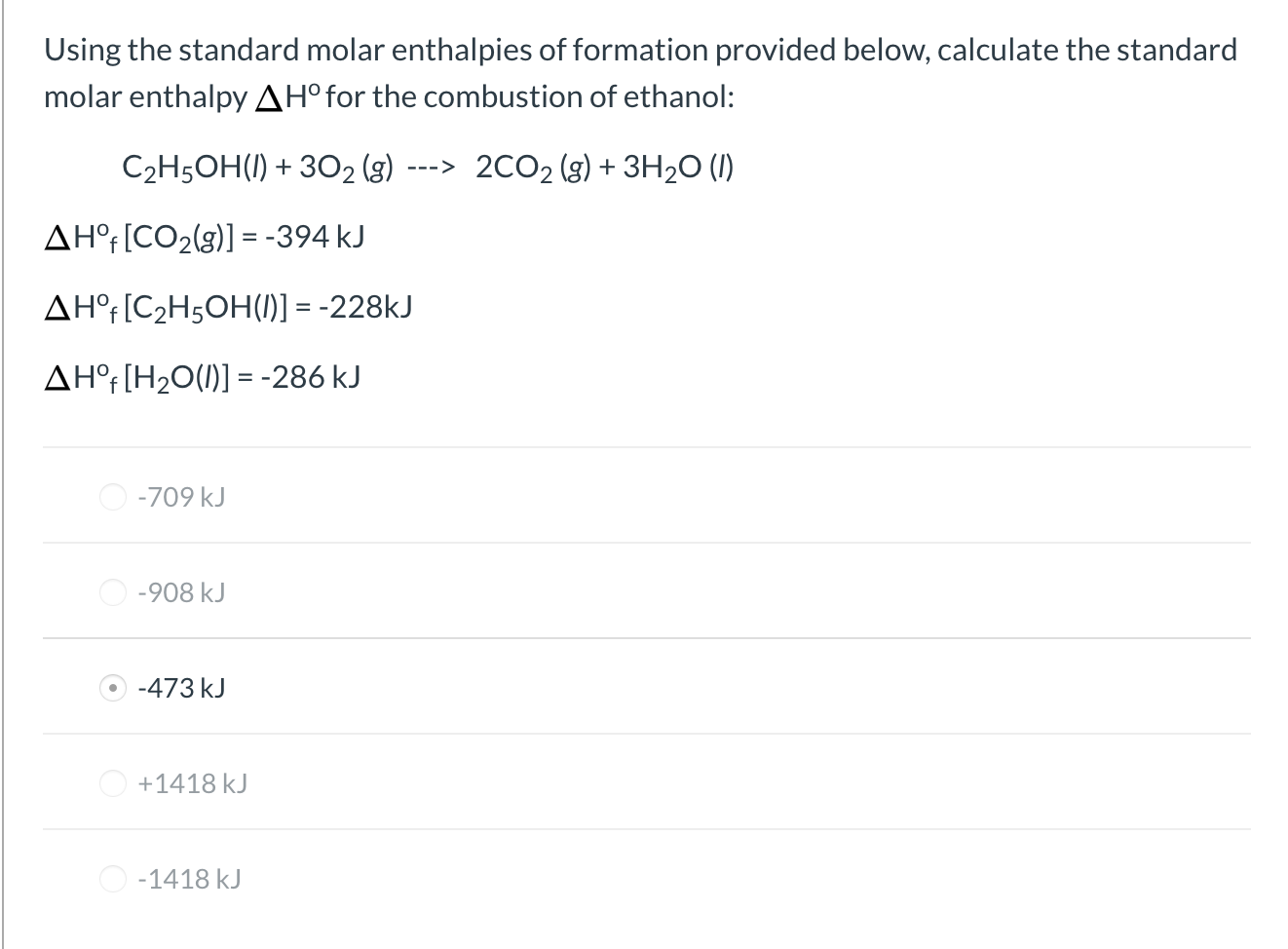
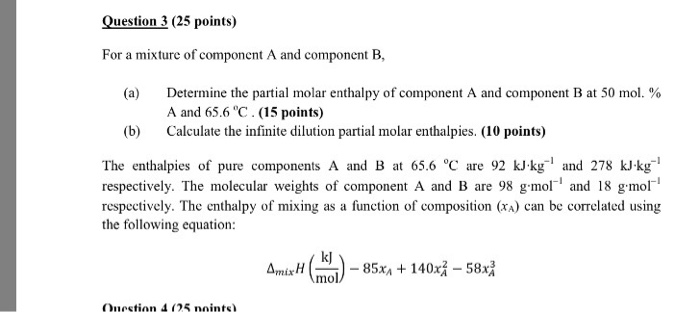
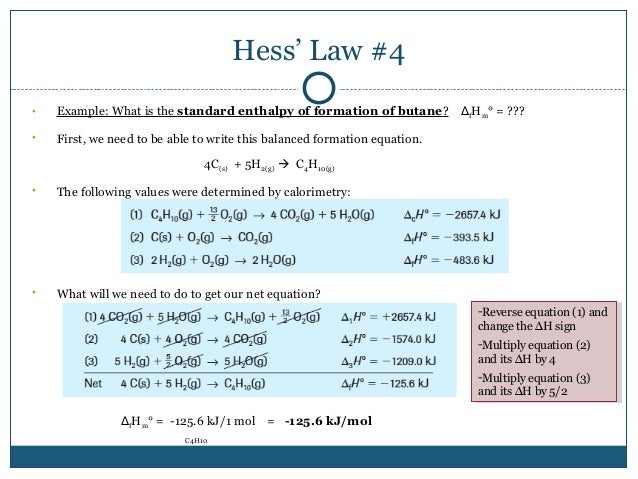
So we convert the carefully measured mass in to moles by dividing by molar mass. Reaction enthalpies are given for two reactions. Any chemical reaction involves two categories of chemicals products and reactants.
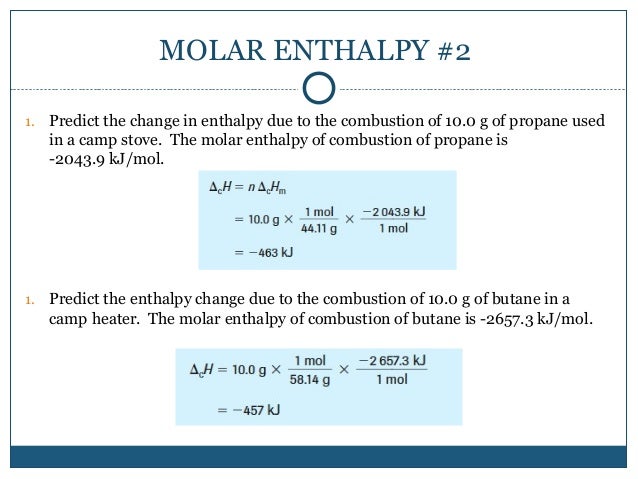
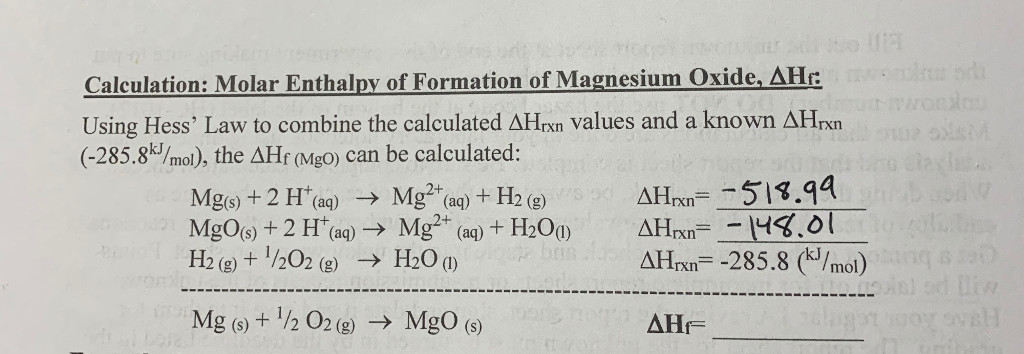
Take the sum of these changes to find the total enthalpy change remembering to multiply each by the number of moles needed in the first stage of the reaction. This is a multiple step problem. 2 the moles is multiplied by the molar heat of solution.
Grab a calculator or use your brain to perform the calculations and obtain the heat capacity of the sample. In an experiment to determine the molar enthalpy of neutralisation 500 ml of 10 mol l 1 naoh aq is placed in the styrofoam cup. Therefore you can find enthalpy change by breaking a reaction into component steps that have known enthalpy values.
4 the is determined from. 3 the joules of heat released in the dissolving process is used with the specific heat equation and the total mass of the solution to calculate the. How to calculate the enthalpy of a chemical reaction.
Next find the masses of your reactants. N number of moles of reactant. 1 the grams naoh is converted to moles.
Hesss law also known as hesss law of constant heat summation states that the total enthalpy of a chemical reaction is the sum of the enthalpy changes for the steps of the reaction. C concentration in m molesl. Determine the total mass of the reactants.
Use the following data. V volume in litres. 3267 2364 858.