How To Figure Out Molar Mass Of An Element
Finding molar mass also called molecular weight molecular mass and gram formula mass is an essential skill in chemistry especially for mole to gram conv.

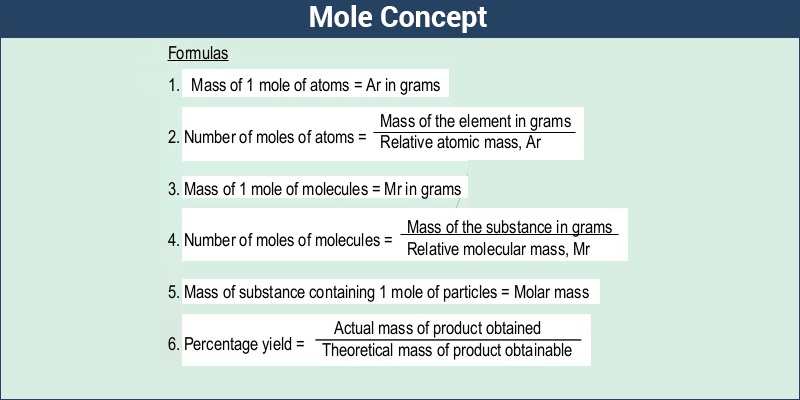
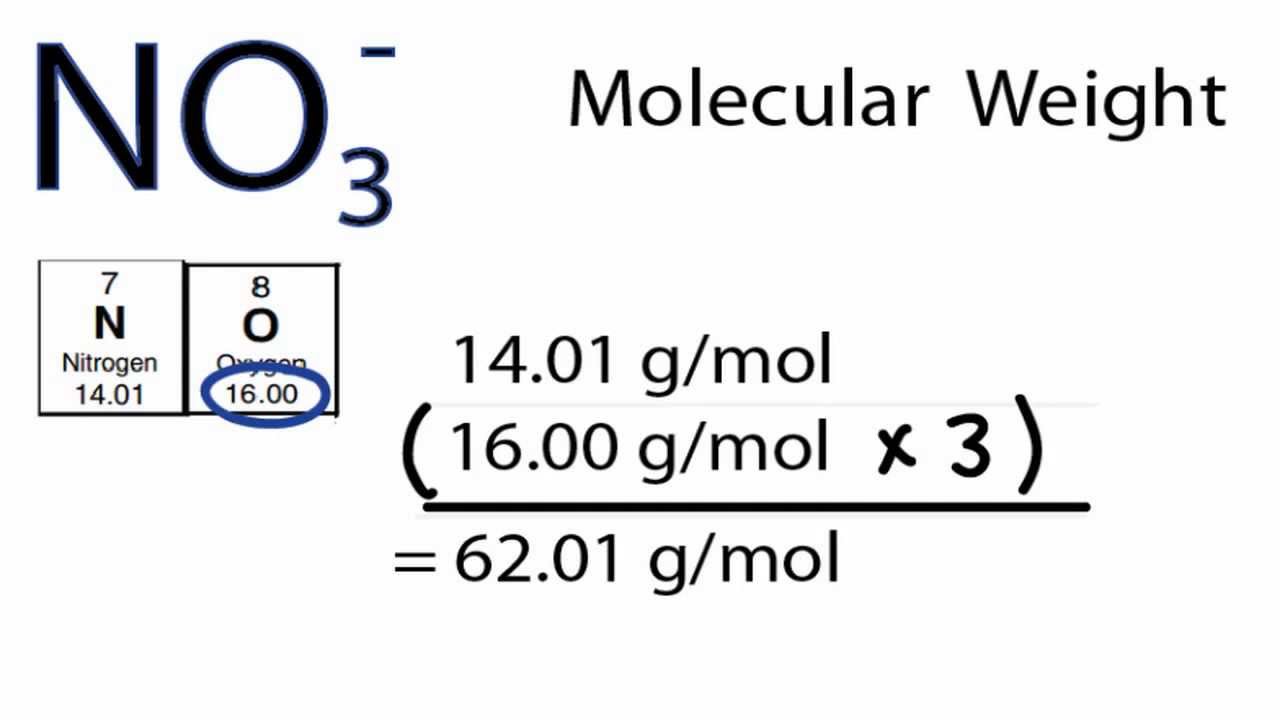
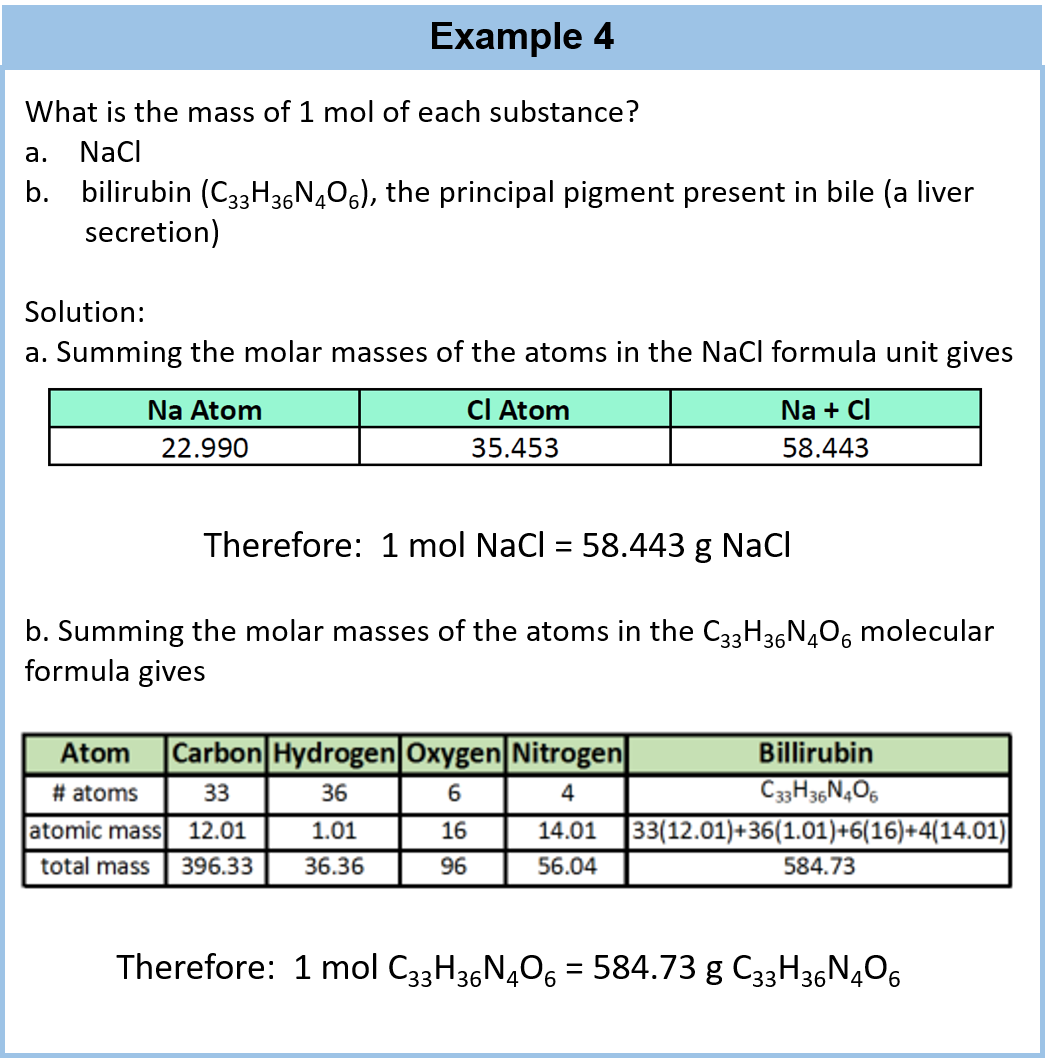
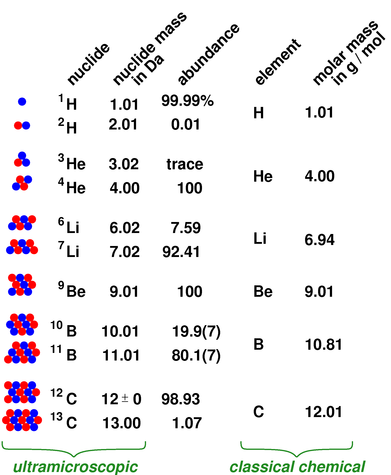
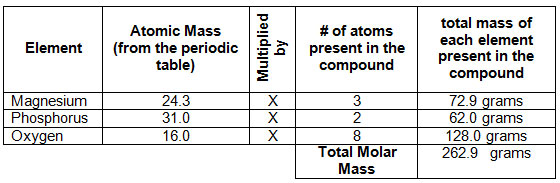
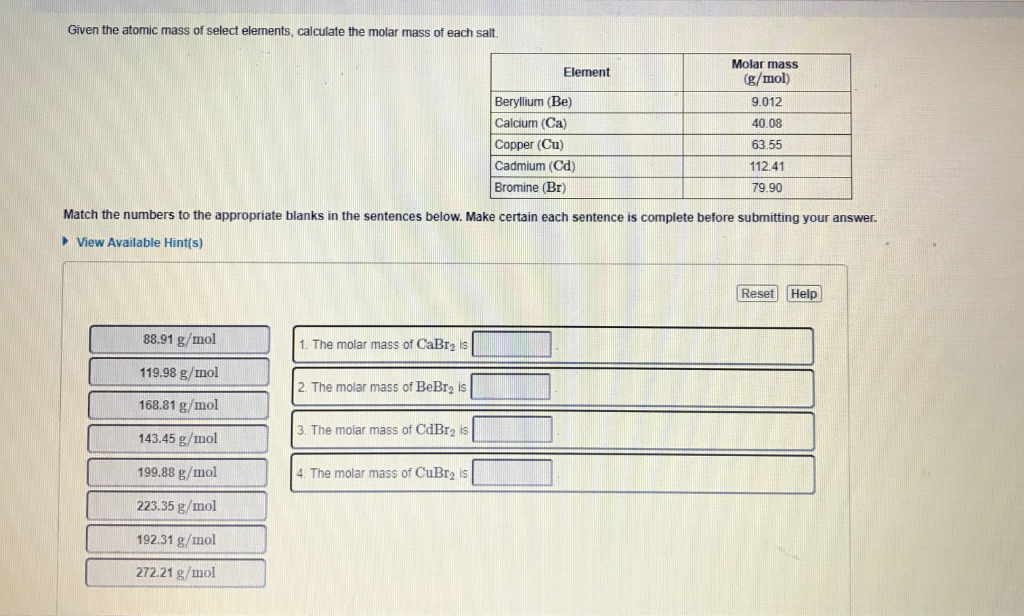
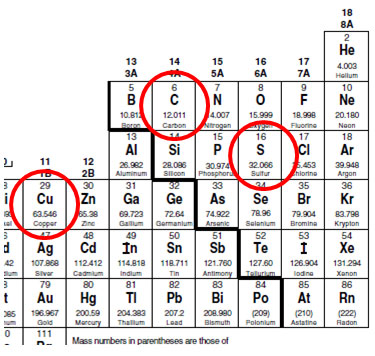
How to figure out molar mass of an element. Take a look at naoh which contains sodium oxygen and hydrogen. Finding the molar mass of a single element is really simple. Multiply the subscript number of atoms times the atomic mass of that element and add the masses of all of the elements in the molecule to get the molecular mass.
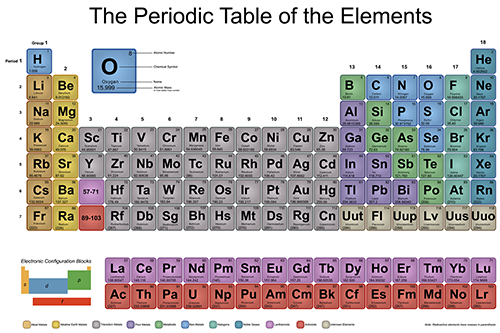
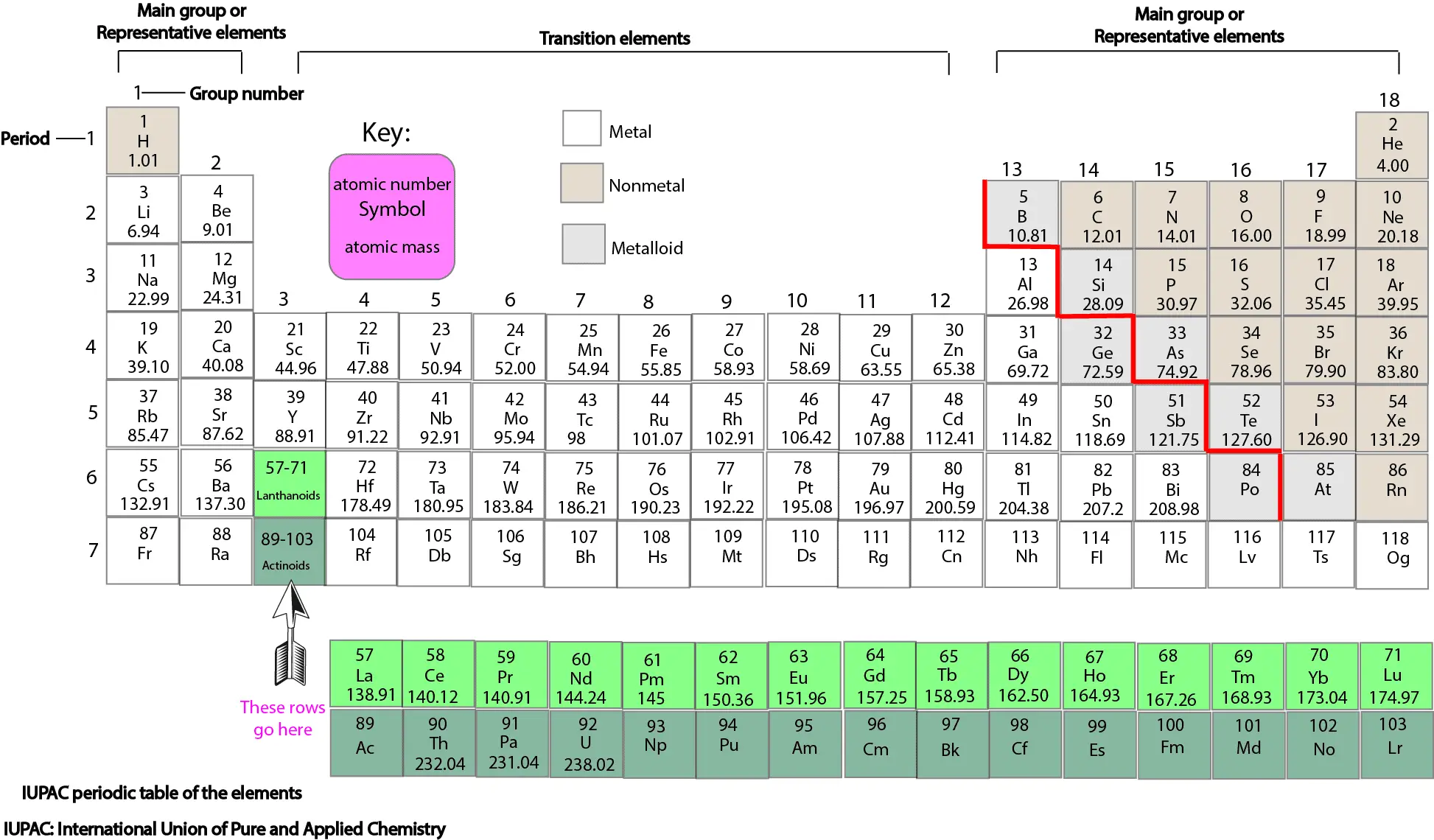
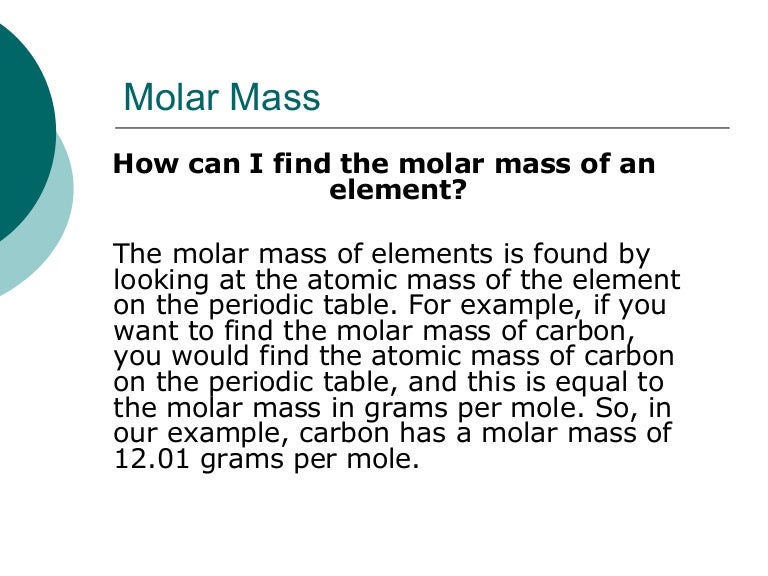
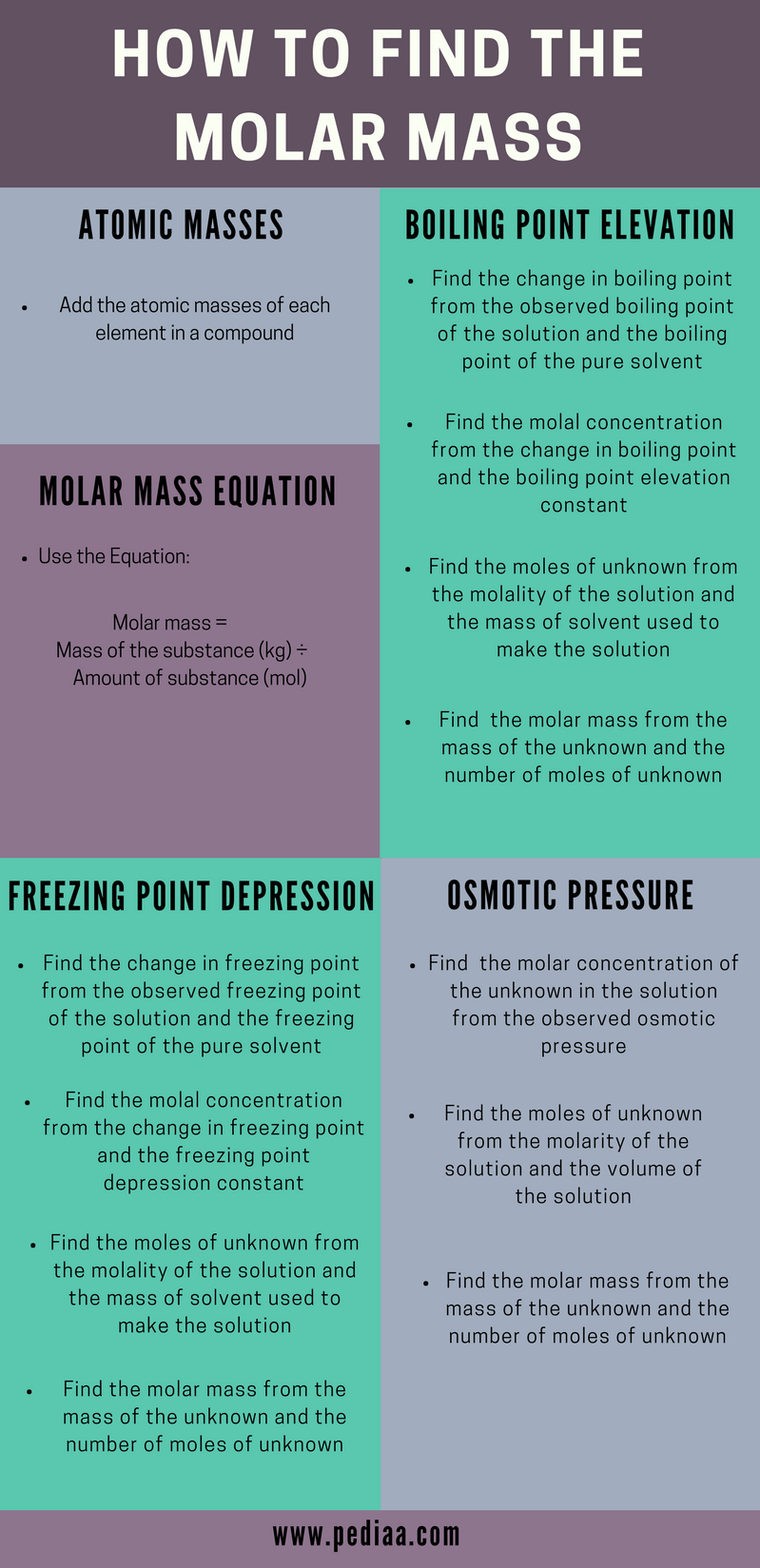
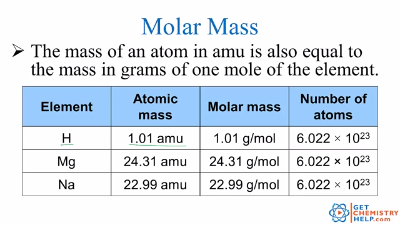
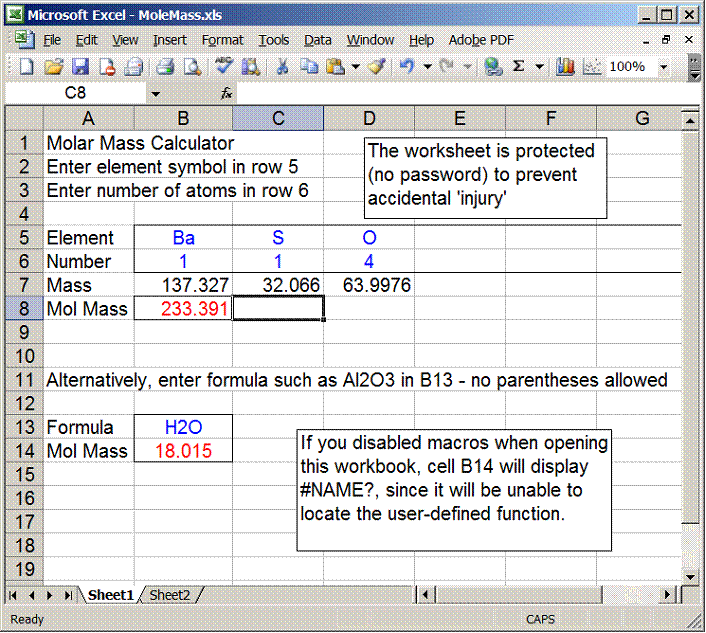
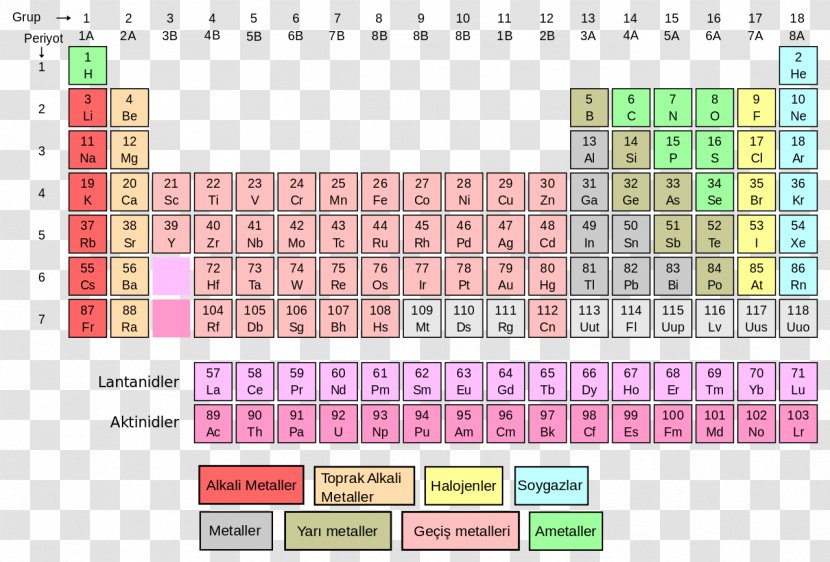
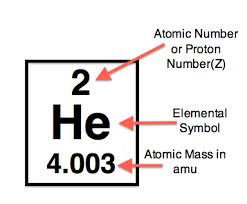
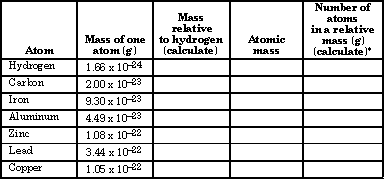
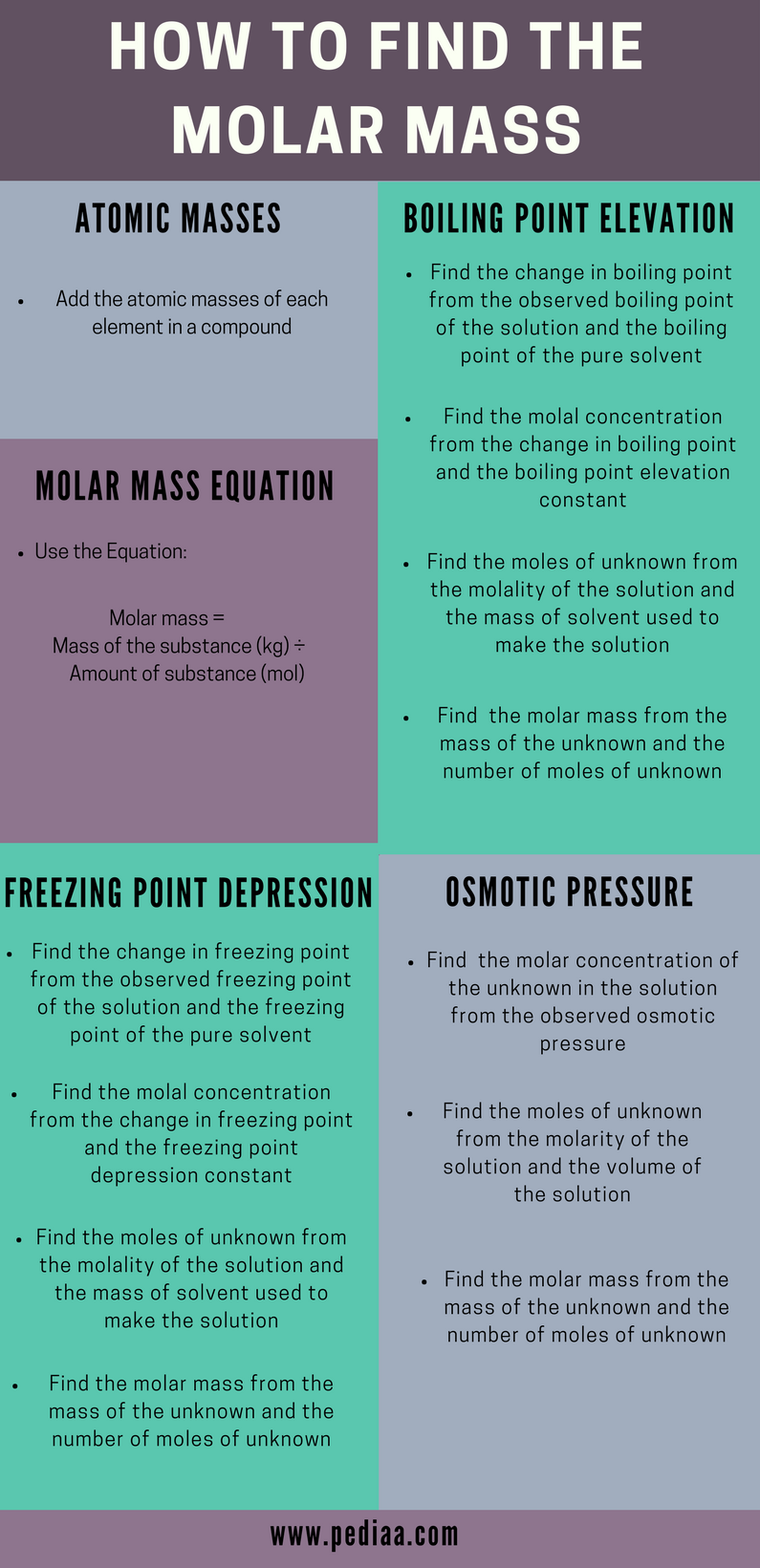
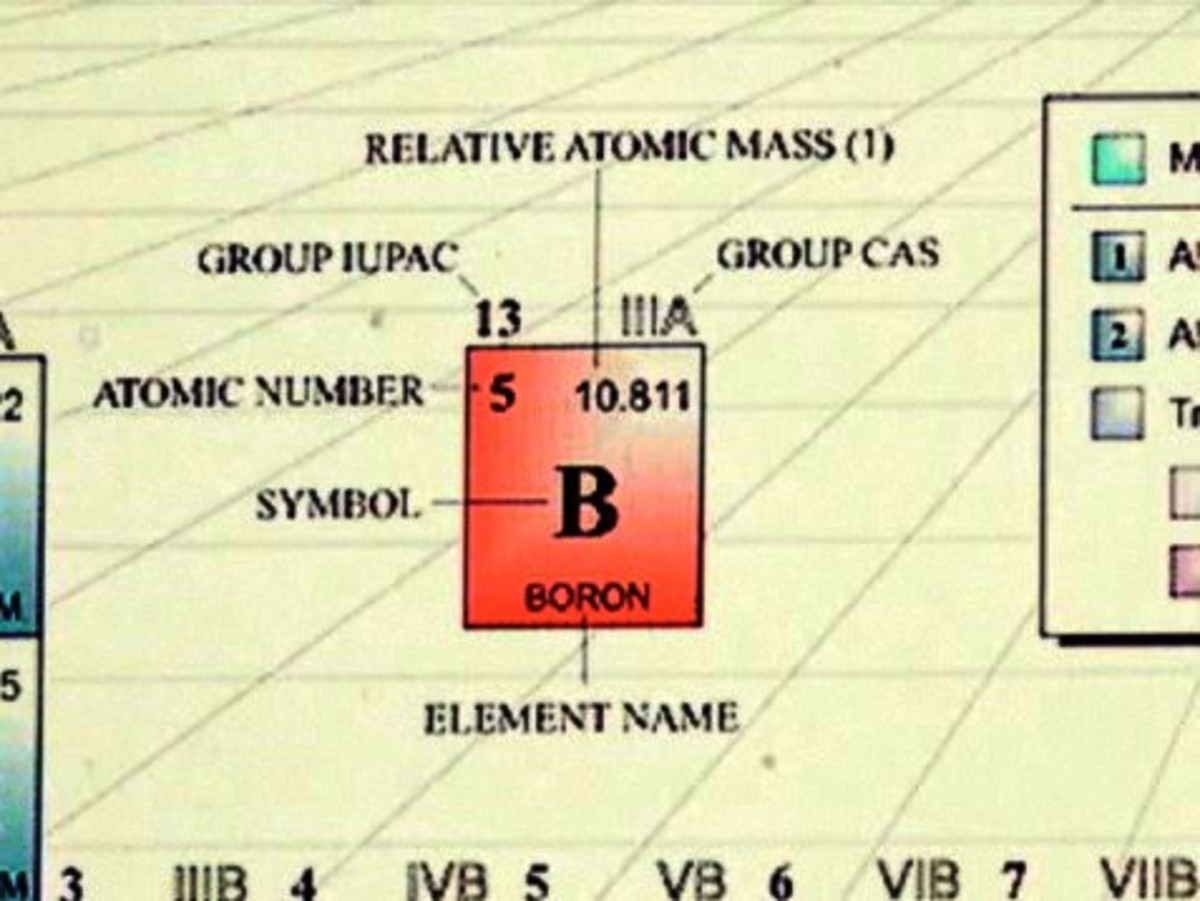
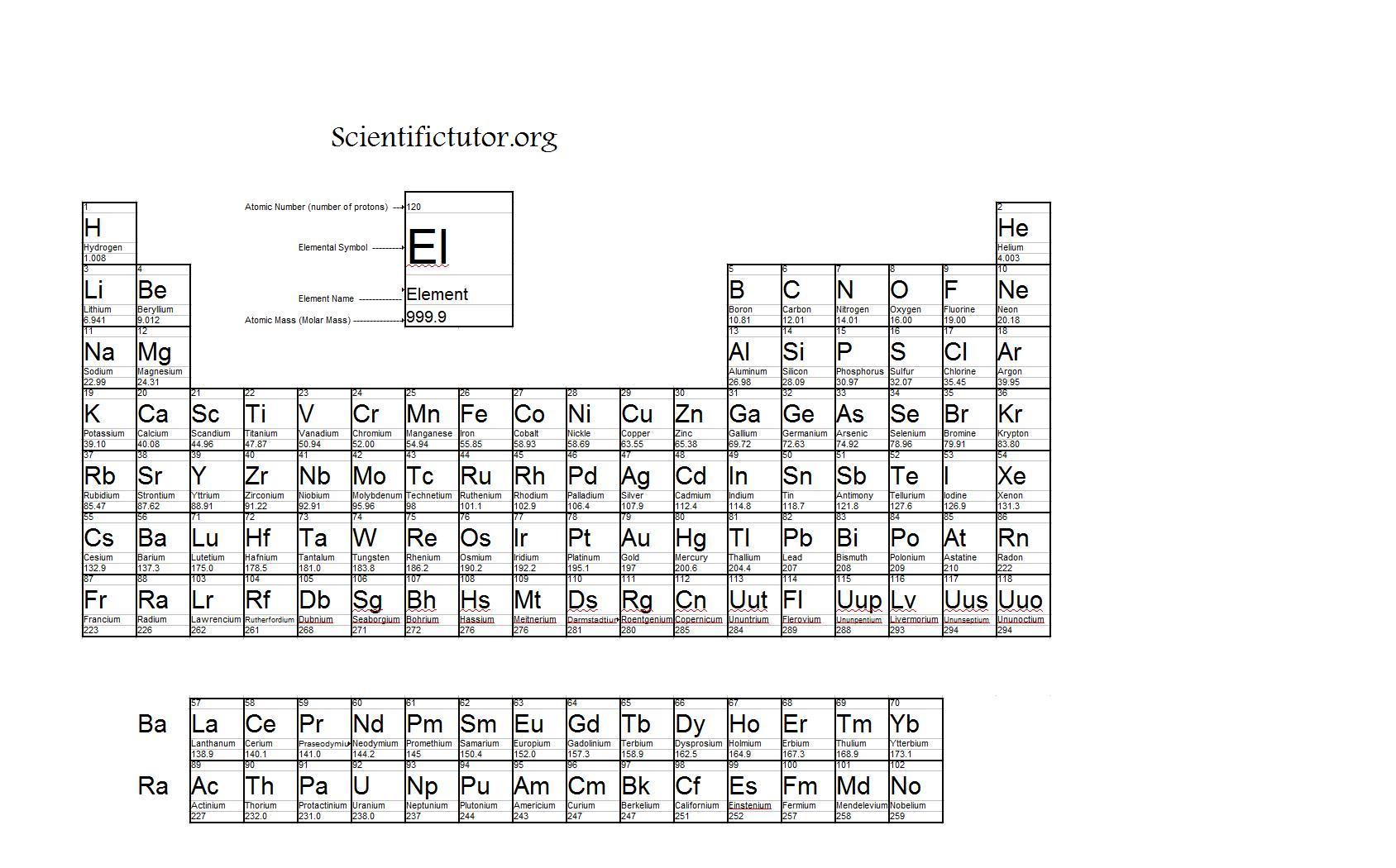
Molar mass usually is expressed in grams g or kilograms kg. To calculate the molar mass of an element find the relative atomic mass of that element then multiply the relative atomic mass by the molar mass constant. All you need to do is find the atomic mass of the element on the periodic table and report the number with the unit grams per mole or gmol.
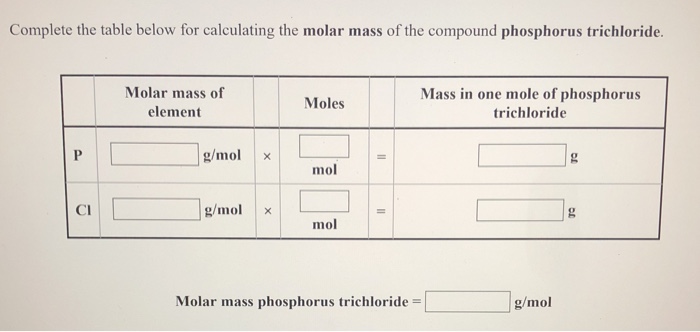
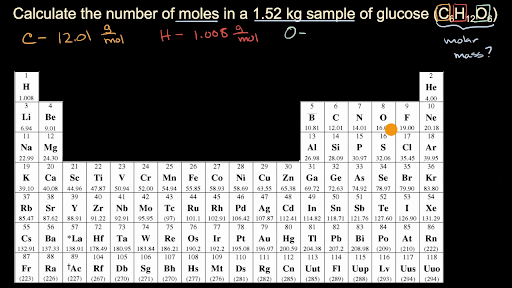
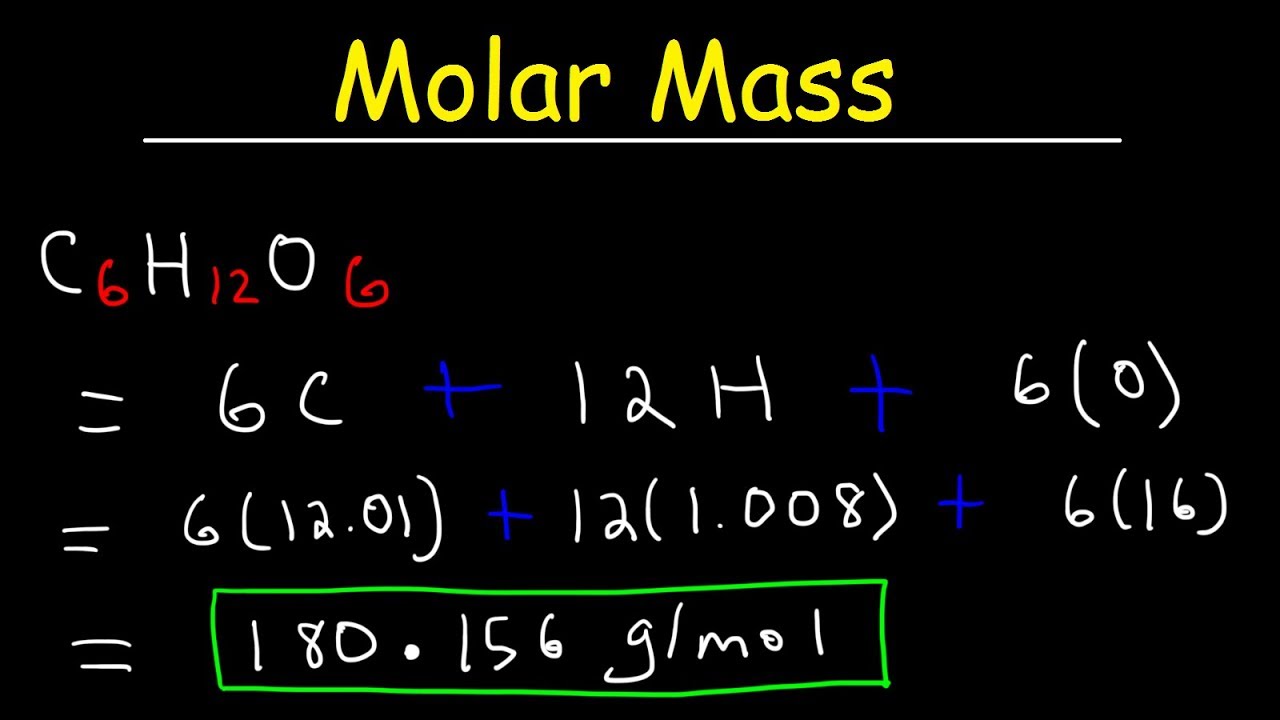
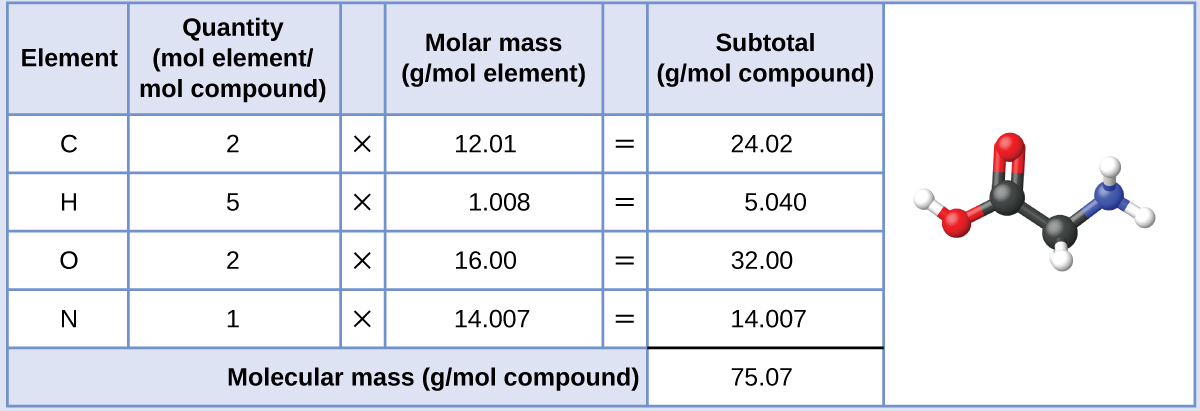
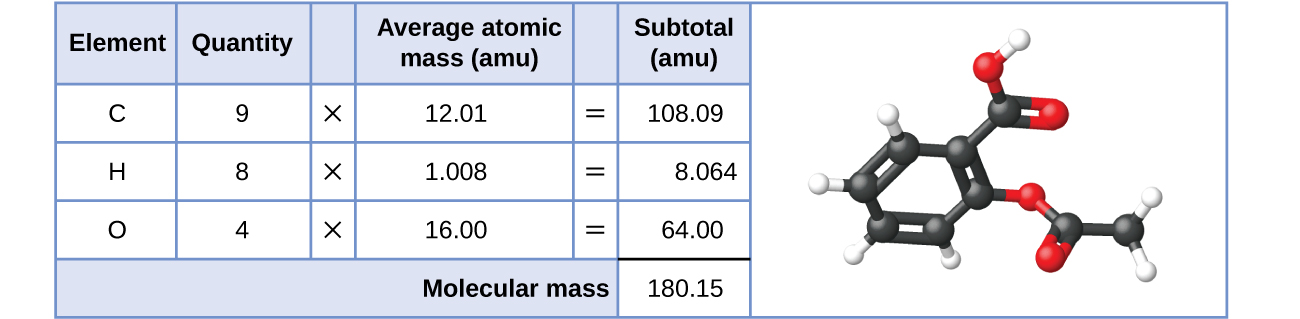
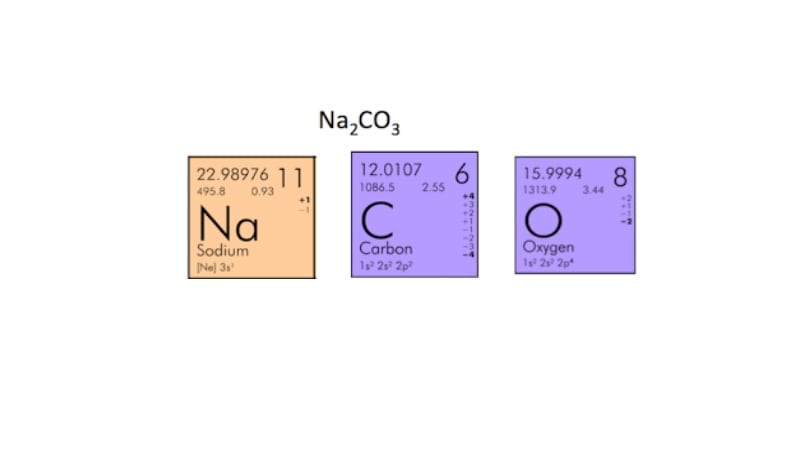
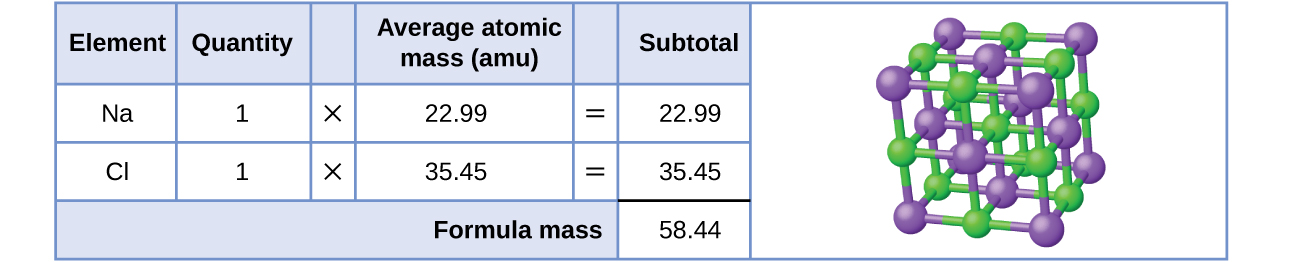
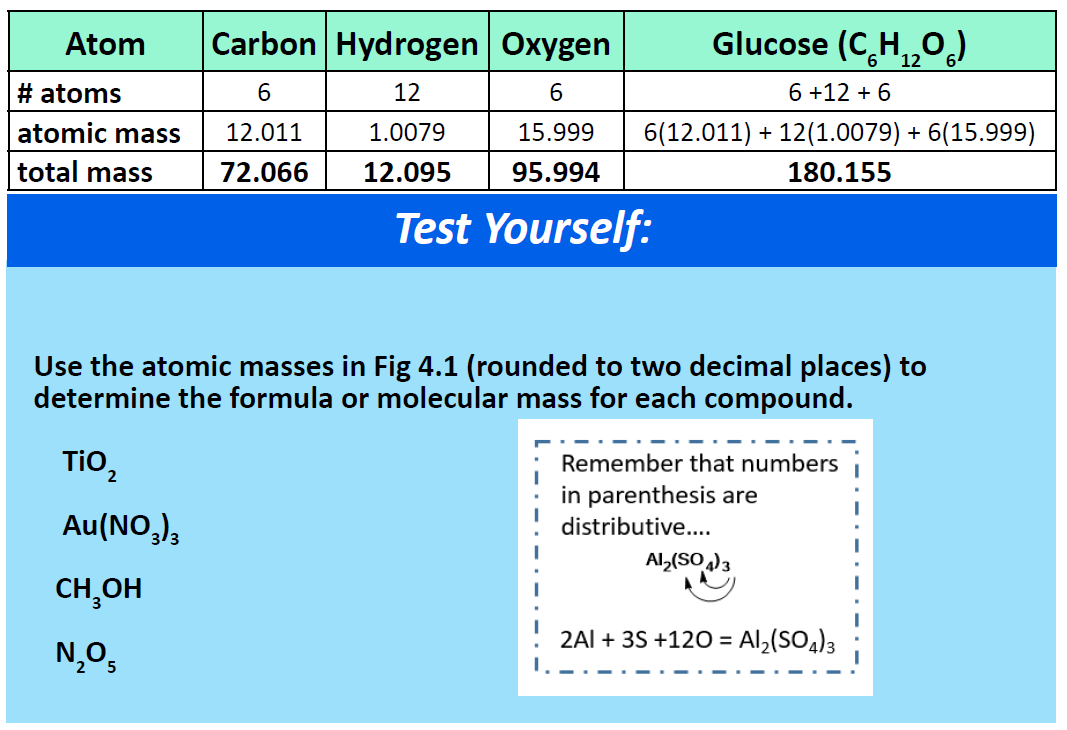
To calculate the molar mass of a compound calculate the molar mass of each element in the compound then multiply the elements atomic mass by the number of atoms of that element in the compound. Adding them together yields.
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcqfcqpxjggp2sliqqfx Tj7nzpocoyomw Eitvegmfeffppb0ka Usqp Cau
encrypted-tbn0.gstatic.com
















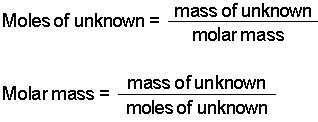



:max_bytes(150000):strip_icc()/GettyImages-175532236-c614b233b7e84d5487cad8b280f365a4.jpg)




















/mass-percent-composition-example-609567_V2-01-89c18a9d30ea43b494d09b81f7ffefc1.png)



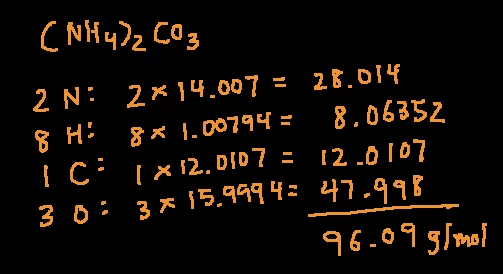










/mass-percent-composition-example-609567_V2-01-89c18a9d30ea43b494d09b81f7ffefc1.png)