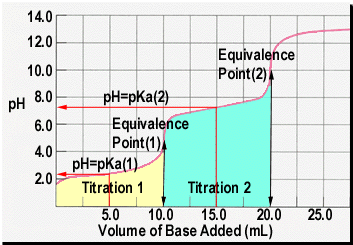
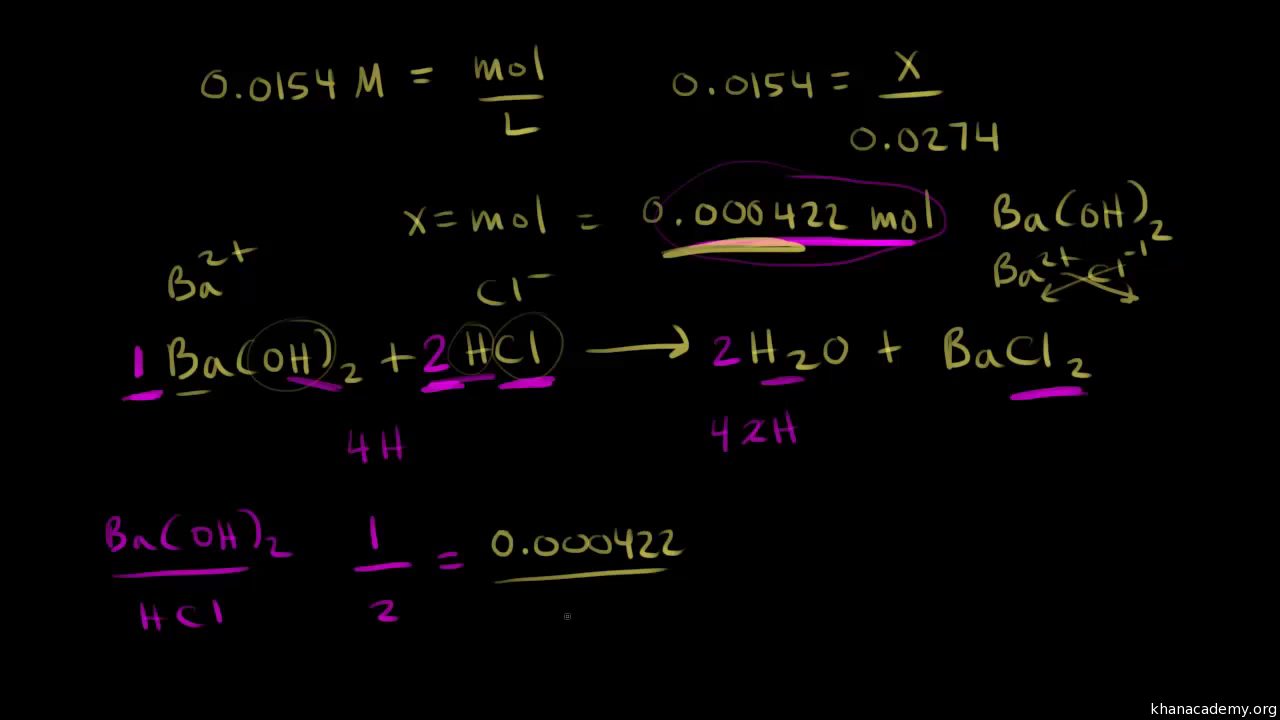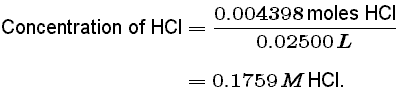How To Find Concentration From Titration
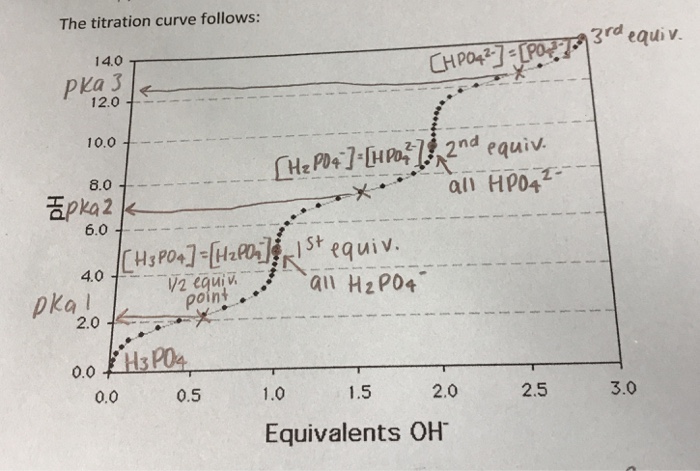
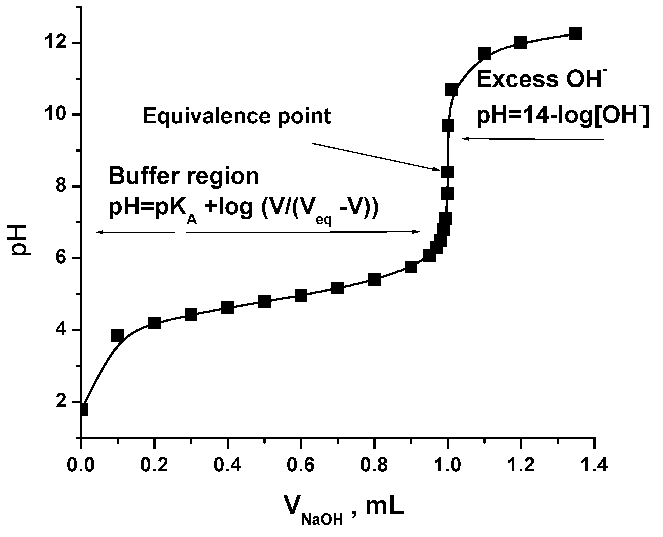
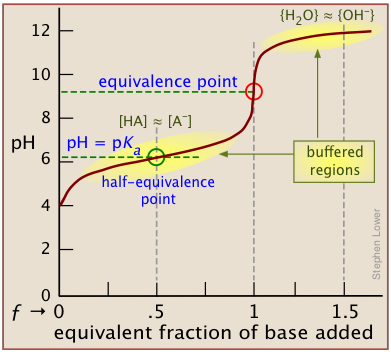
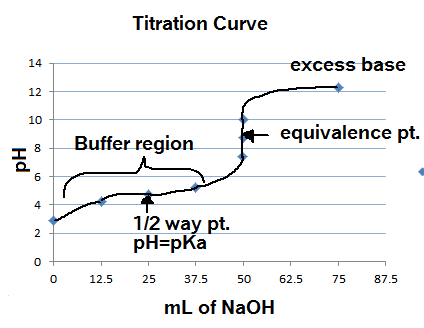
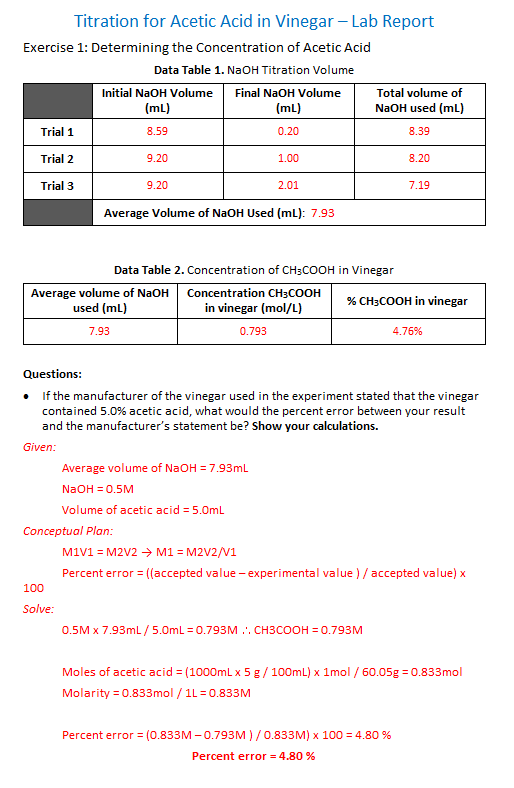
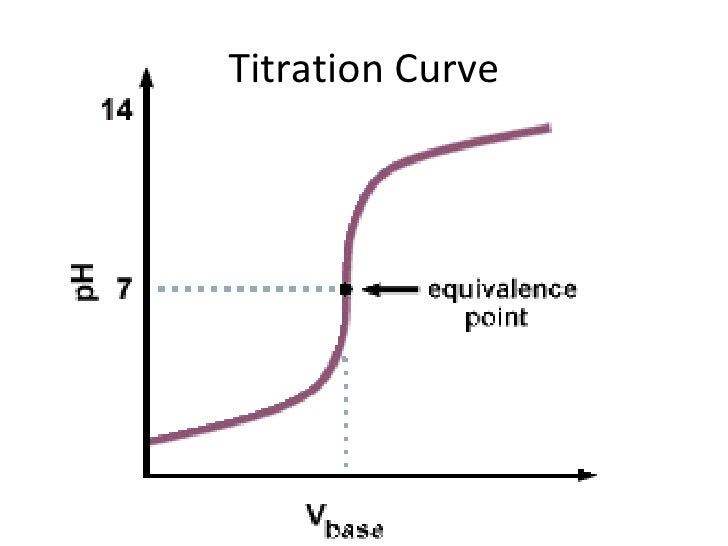
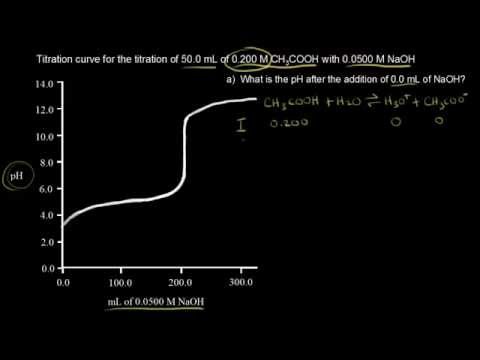
The moles of acid will equal the moles of the base at the equivalence point.

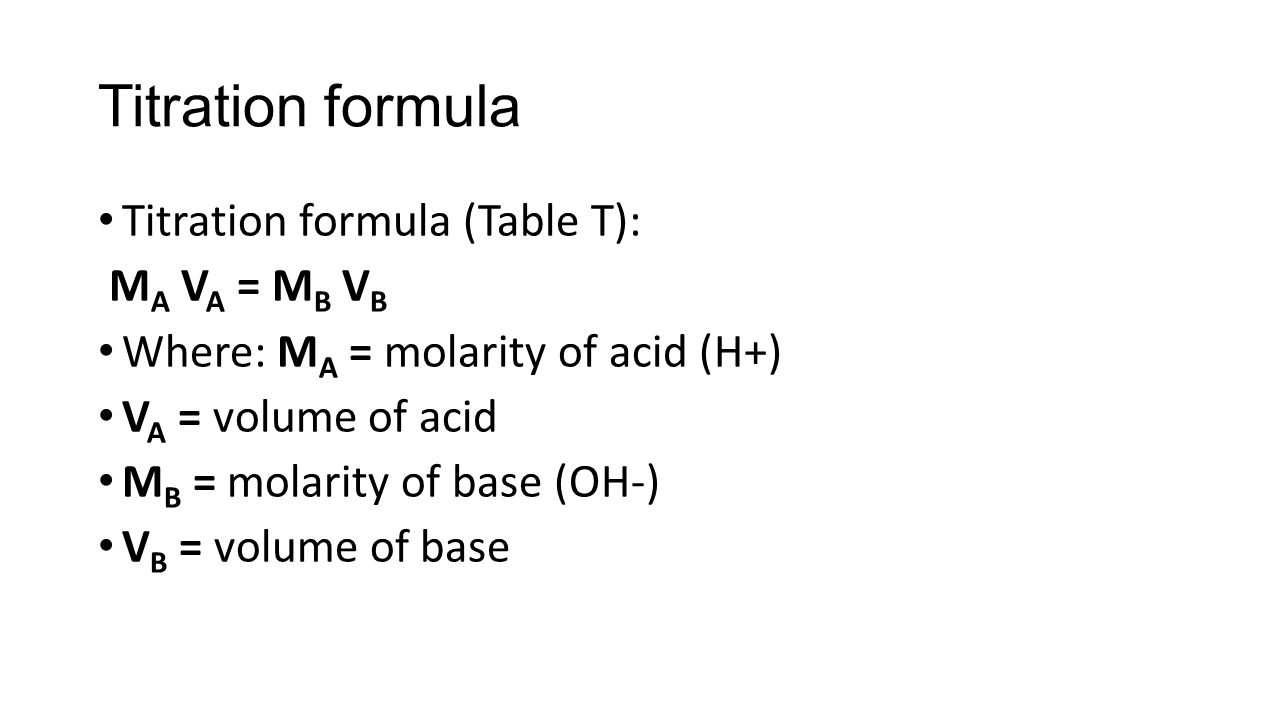
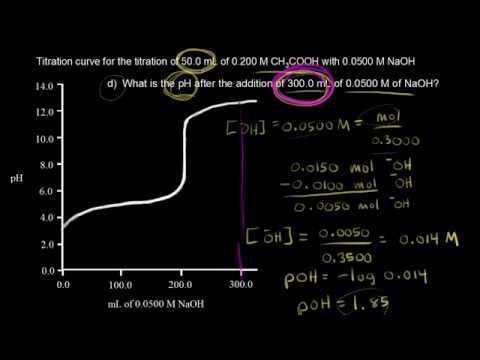
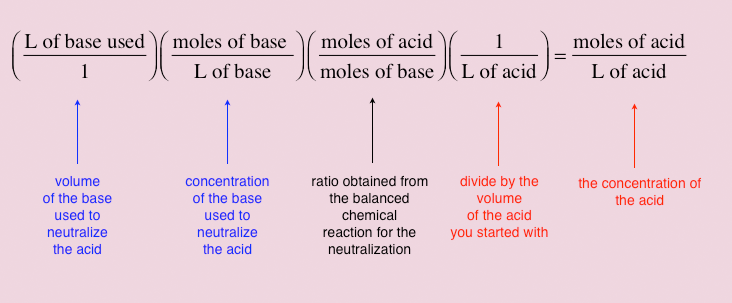
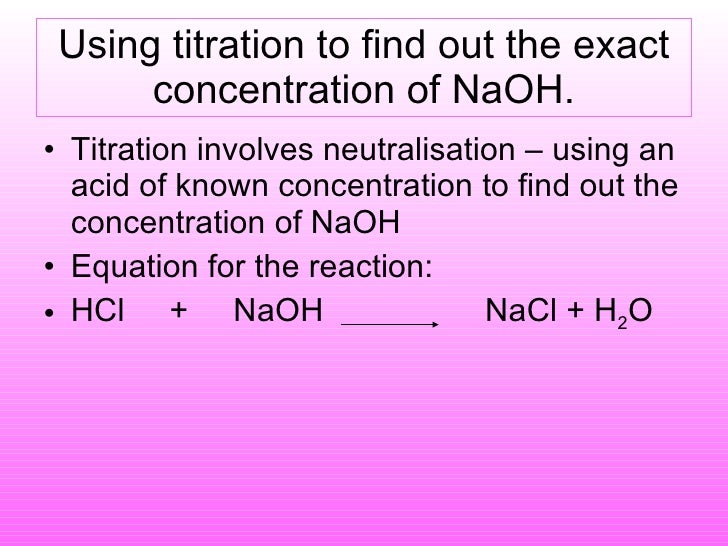
How to find concentration from titration. Use the titration formula. Determine oh every mole of naoh will have one mole of oh. M av a m bv b.
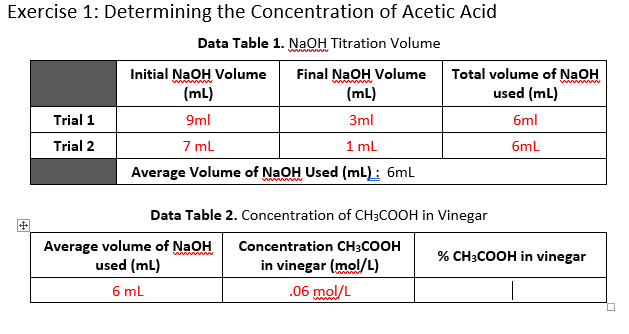
Molarity is the concentration of a solution expressed as the number of moles of solute per litre of solution. Therefore oh 05 m. Titrations are commonly used to determine the concentration of acid rain that falls.
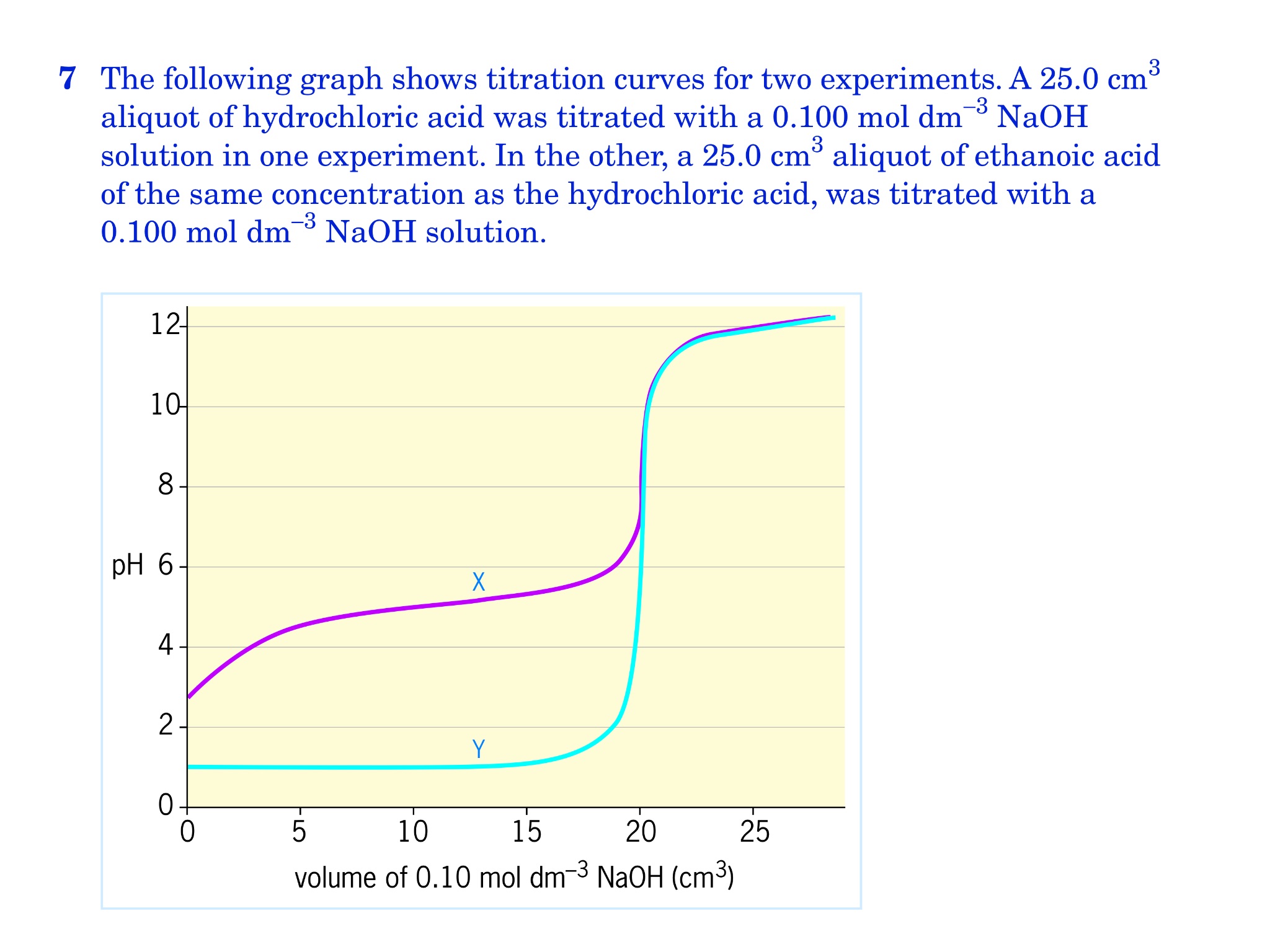
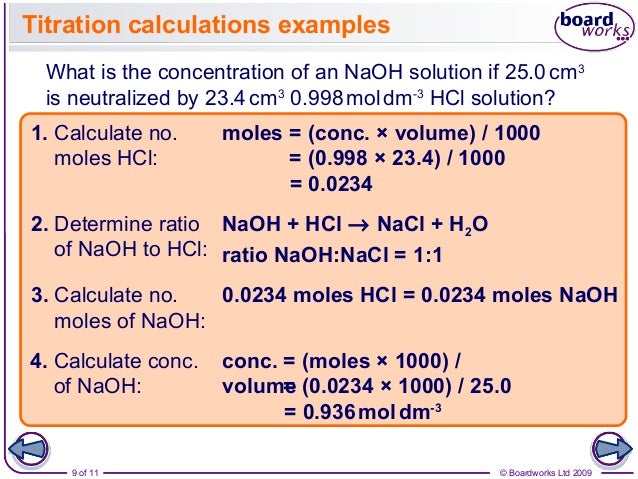
If you solve for m a you will see that. In a titration 250 cm3 of 0100 moldm3 sodium hydroxide solution is exactly neutralised by 2000 cm3 of a dilute solution of hydrochloric acid. M a 25m hcl.
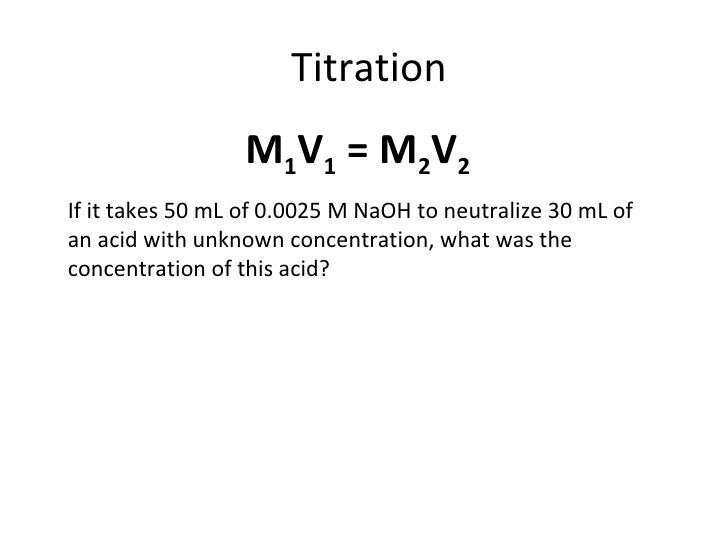
So we have 200 milliliters of hcl and this time instead of using sodium hydroxide were going to use barium hydroxide and it takes 274 milliliters of a 00154 molar solution of barium hydroxide to completely neutralize the acid thats present. Suppose that a titration is performed and 2070 ml of 0500 m naoh is required to reach the end point when titrated against 1500 ml of hcl of unknown concentration. Heres how to perform the calculation to find your unknown.
Voiceover lets do another titration problem and once again our goal is to find the concentration of an acidic solution. To do this a small sample is titrated to find its acidity which tells us how much base we need to neutralise the batch successfully. Number of moles oh 05 m 0.
It takes 25ml of naoh to neutralize the acid. So if you know one value you automatically know the other. Lets assume you are titrating a strong acid 10 ml unknown concentration hcl with a strong base 10 m naoh.
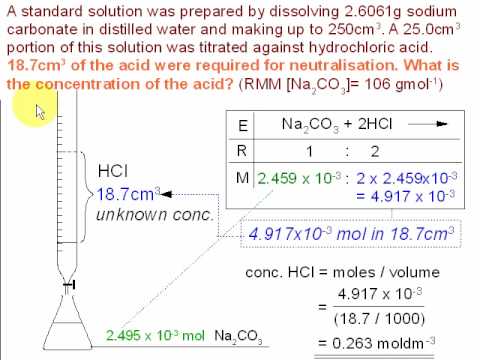
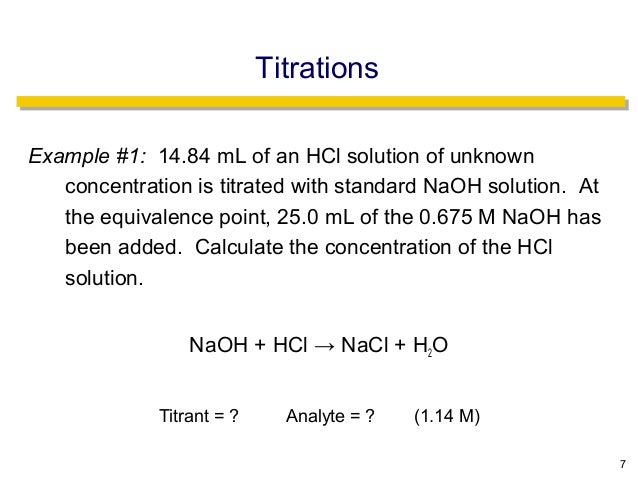
The addition of a base removes the free fatty acids present which can then be used to produce soap. Calculate the concentration of the hydrochloric. The technique involves determining accurately the volume of the standard solution needed to react exactly with a known volume of another solution contained in a conical flask in a reaction for which the equation stoichiometry is known.
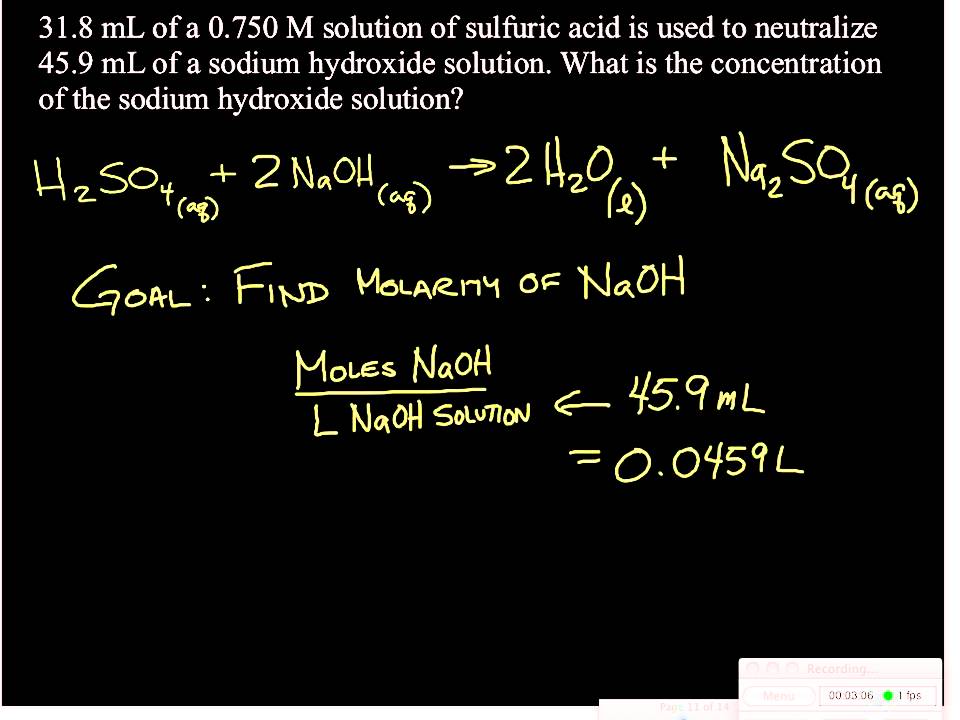
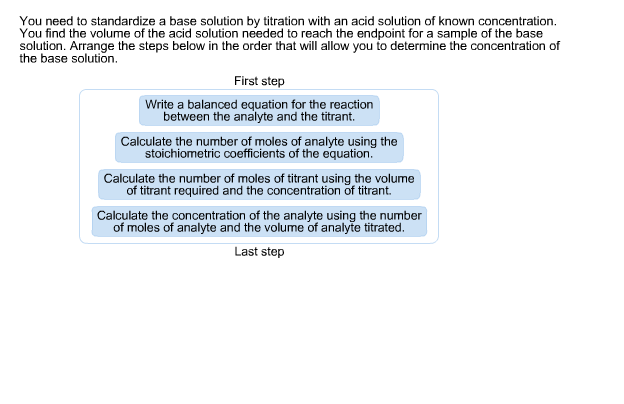
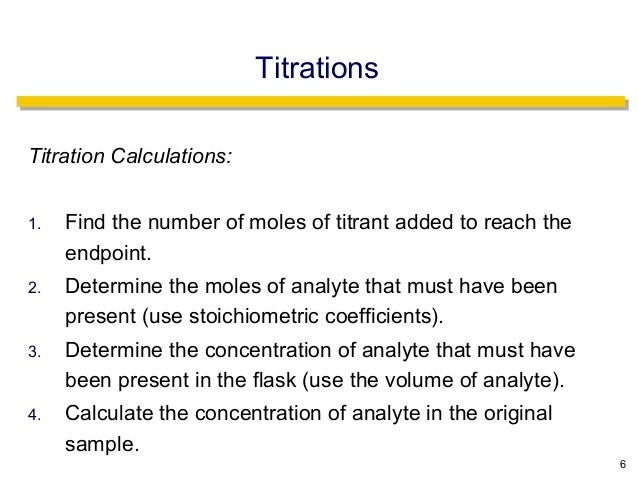
21184 m a m b v b v a 0500 m 2070 ml 1500 ml 0690 m. Titration problem step by step solution. An acid base titration is a neutralization reaction performed in the lab to determine an unknown concentration of acid or base.
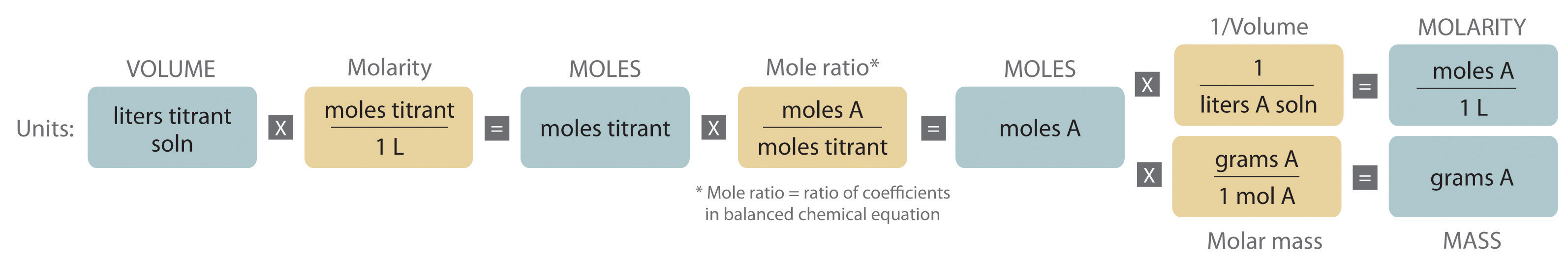
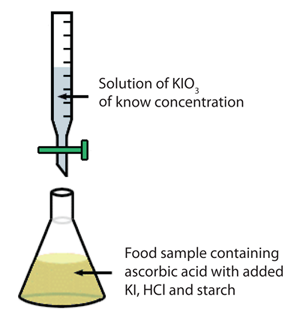
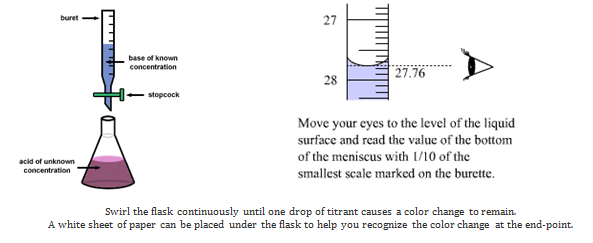
Titration is a very useful laboratory technique in which one solution is used to analyse another solution. One of the solutions is a standard solution of known concentration and is delivered from a burette. The above equation can be used to solve for the molarity of the acid.
Determine the number of moles of oh molarity number of molesvolume number of moles molarity x volume.

Exp 10 Vinegar Analysis Acid Base Titrations Purpose To Use Quantitative Analysis And Titrations To Find The Concentration Of An Acid Or Base In This Ppt Download
slideplayer.com







/how-to-calculate-normality-609580final2-0d5efa5a961f4fa0a7efc780921faee1.png)























.PNG)