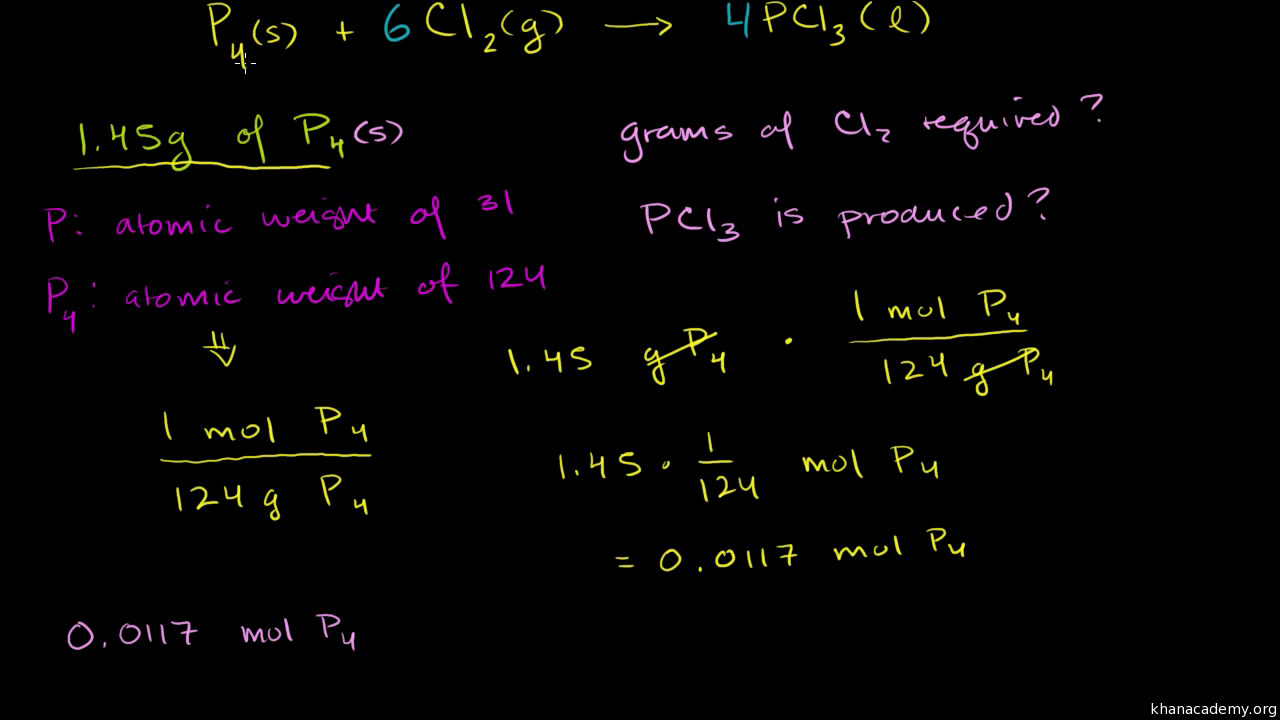How To Find Percent Yield Of A Decomposition Reaction
The actual yield will always be less than the theoretical yield because no chemical reaction ever reaches 100 percent completion.

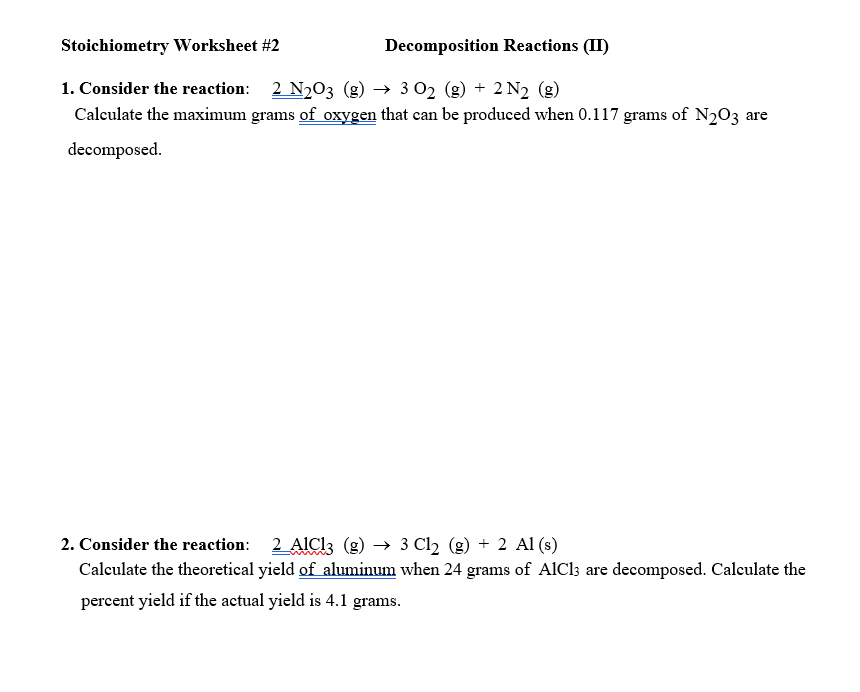
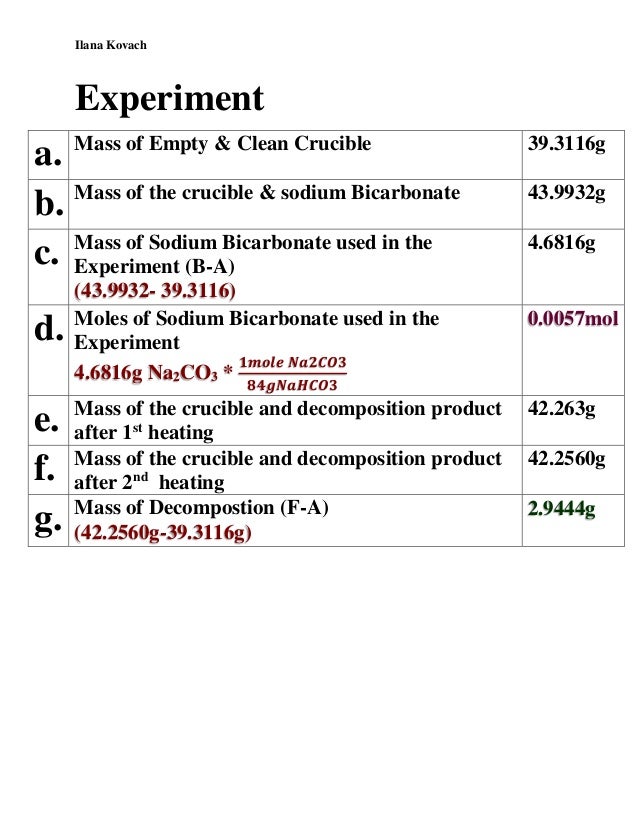
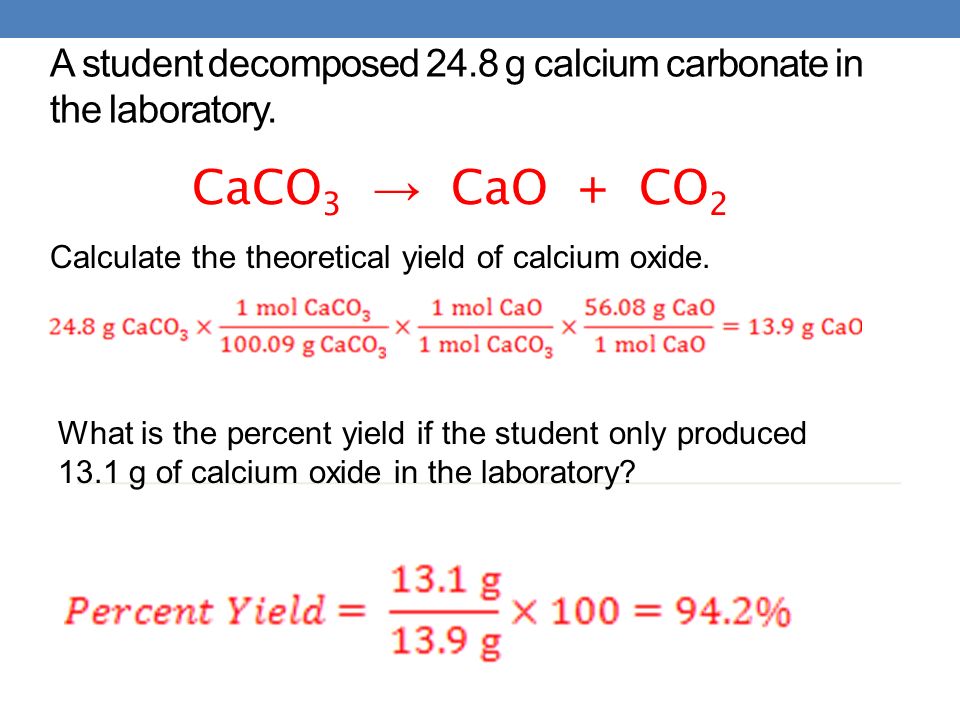
How to find percent yield of a decomposition reaction. Percent yield 79. In a lab setting theres always some amount of error whether its big or small. The actual mass of.
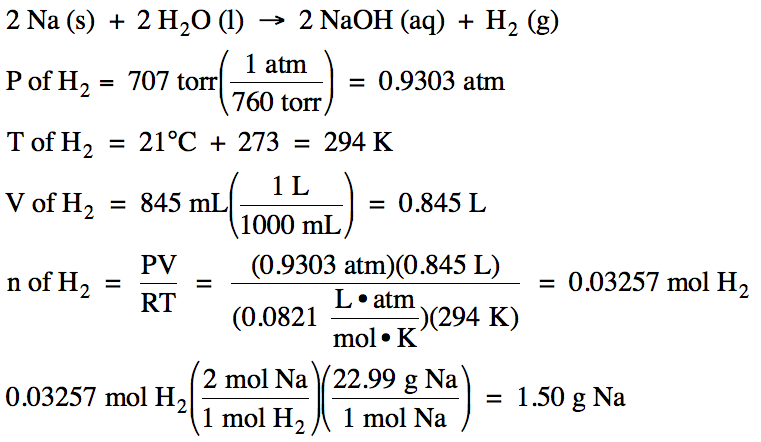
Yield actual yieldtheoretical yield x 100. In this equation the reactant and the product have a 11 mole ratio so if you know the amount of reactant you know the theoretical yield is the same value in moles not grams. Percent yield 15 g 19 g x 100.
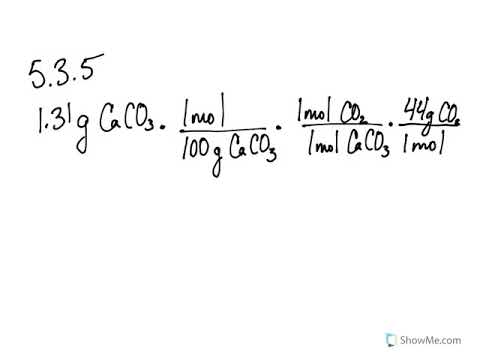
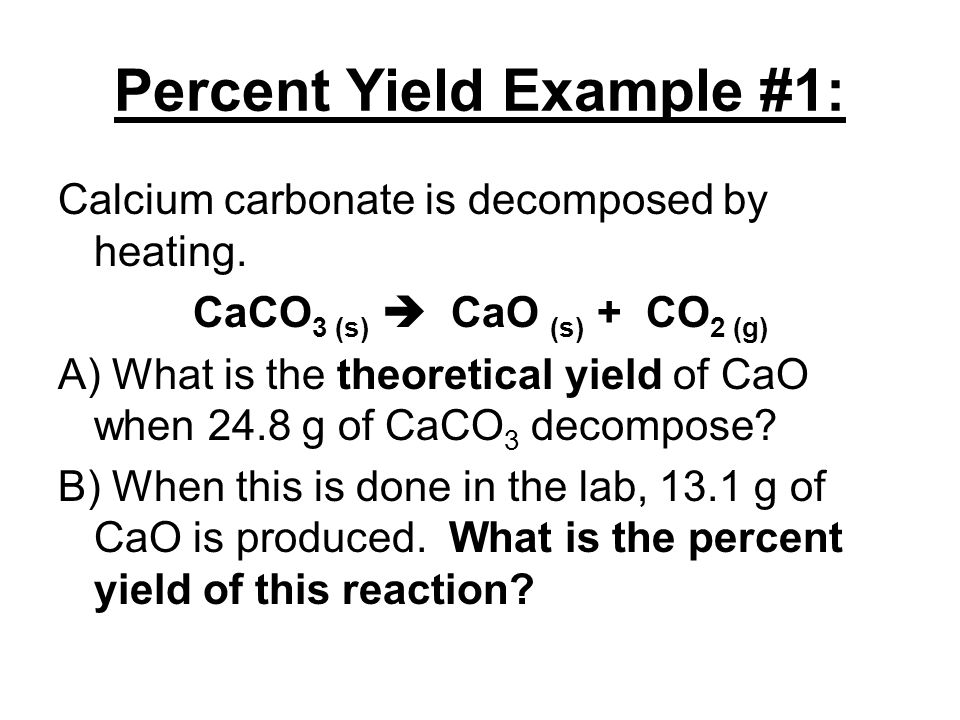
So the percent yield 1212 1305 100 929. Consider a 352 g sample of caco 3 9987 pure in a flask and a 1000 ml sample of vinegar 5 acidity in a graduated cylinder. Part 1 finding the limiting reactant.
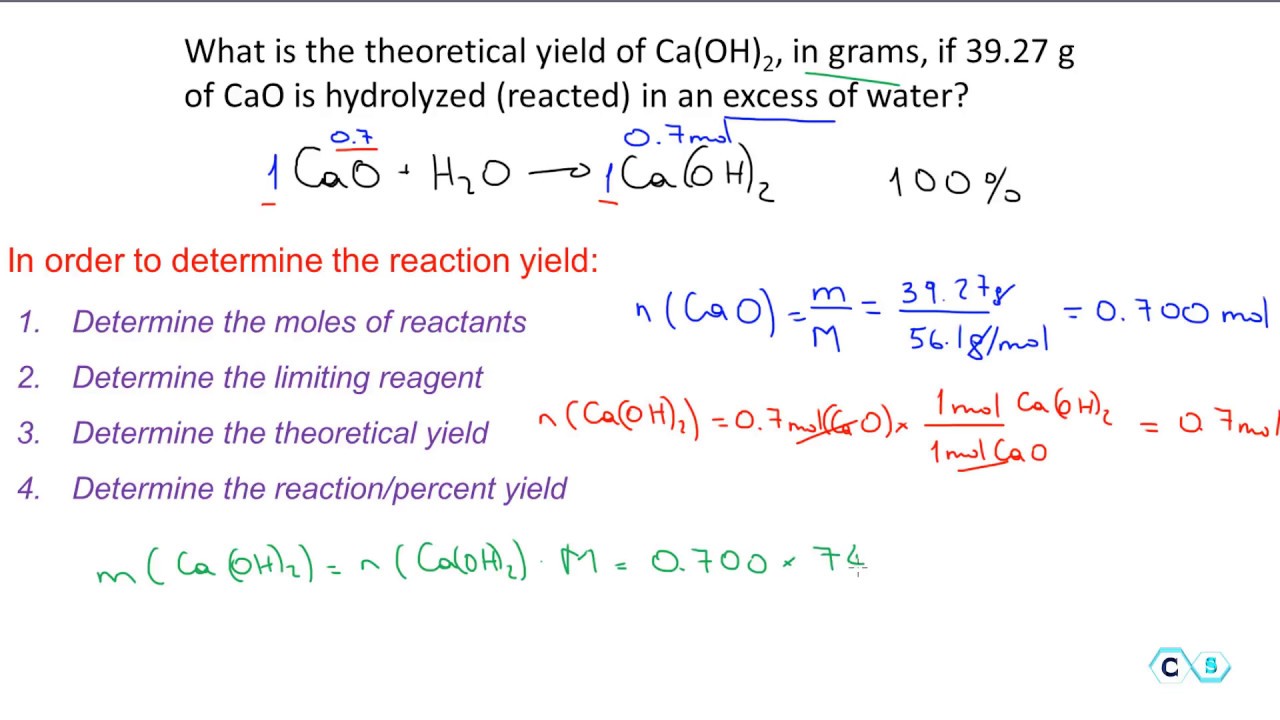
Calculate the percent yield of sodium sulfate when 3218 g of sulfuric acid reacts with excess sodium hydroxide to. The actual yield is the amount of product that is actually formed when the reaction is carried out in the laboratory. Percent yield actual yield theoretical yield x 100.
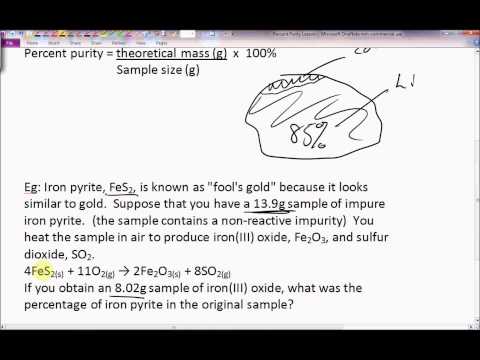
Before performing chemical reactions it is helpful to know how much product will be produced with given quantities of reactants. The percent yield is the ratio of the actual yield to the theoretical yield expressed as a percentage. Percent yield actual yield theoretical yield 100 percent yield is very important in the manufacture of products.
This is known as the theoretical yieldthis is a strategy to use when calculating the theoretical yield of a chemical reaction. A percent yield of 90 means the reaction was 90 efficient and 10 of the materials were wasted they failed to react or their products were not captured. To express the efficiency of a reaction you can calculate the percent yield using this formula.

Calculate The Theoretical Yield To Determine The Yield In A Chemical Reaction Youtube
www.youtube.com































/148302528-56a12f323df78cf77268383a.jpg)