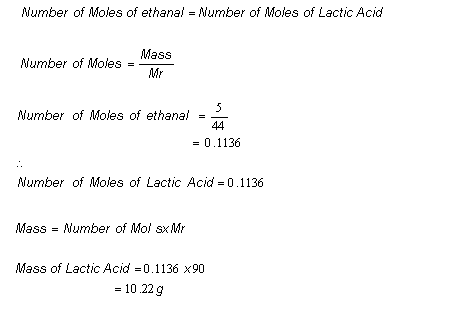How To Find Percent Yield Of A Reaction
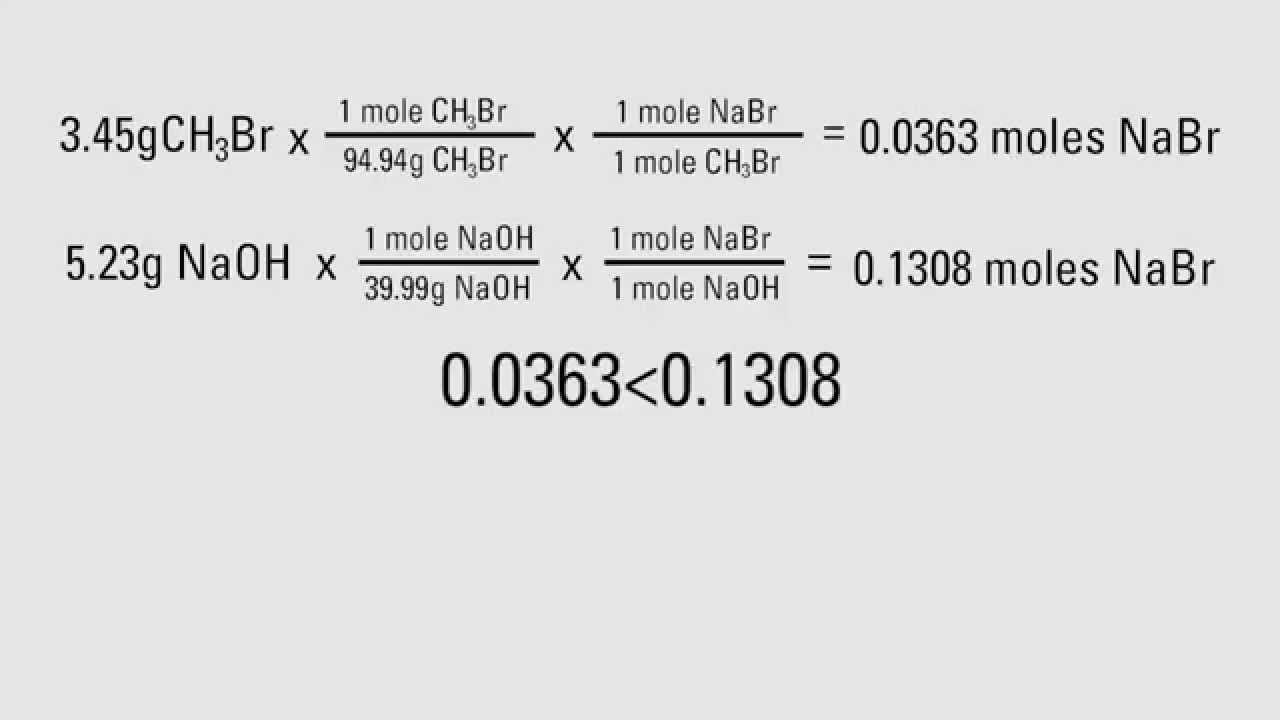
Calculate the molar mass of each reactant.

How to find percent yield of a reaction. Look up the molar mass of each atom in the compound then. With these two pieces of information you can calculate the percent yield using the percent yield formula. Percentage yield is a concept used in chemistry which compares the theoretical yield of an experiment with the actual results observed.
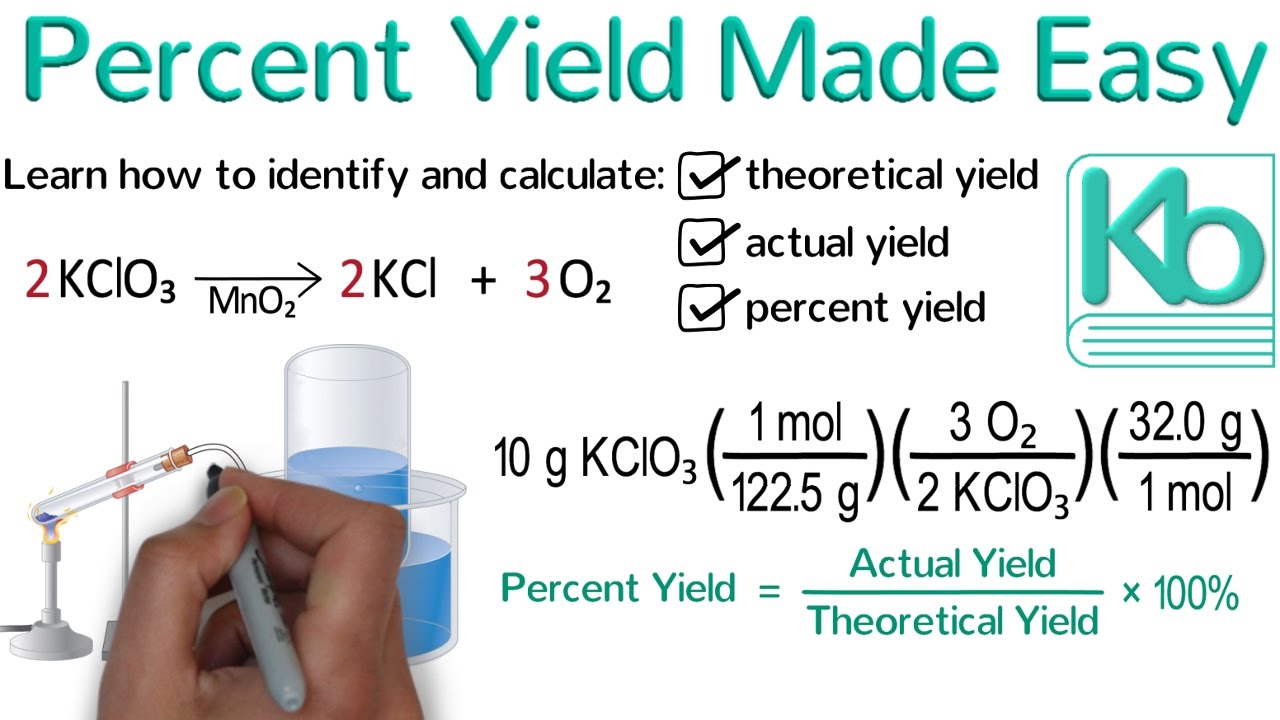
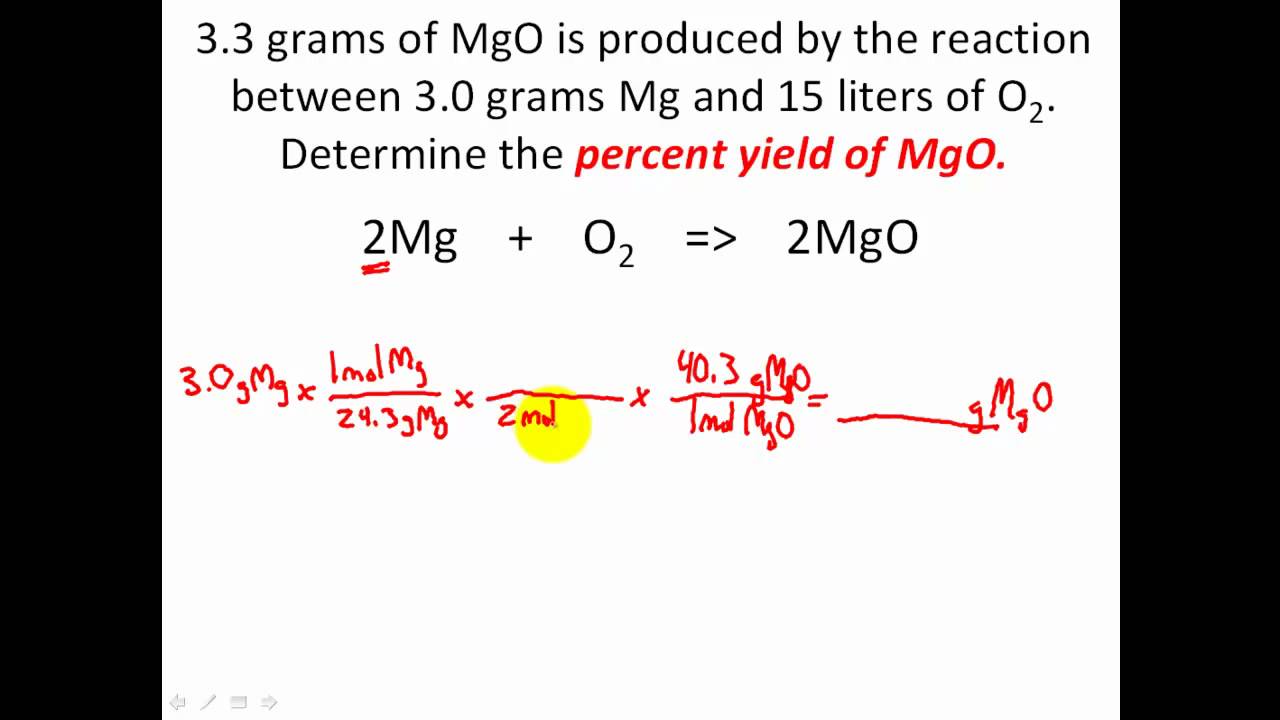
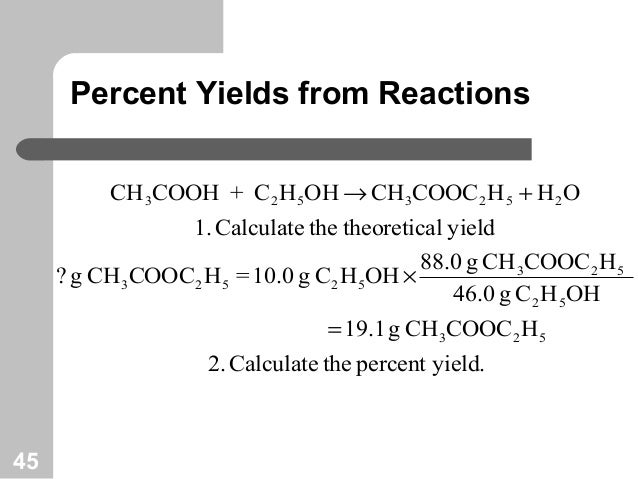
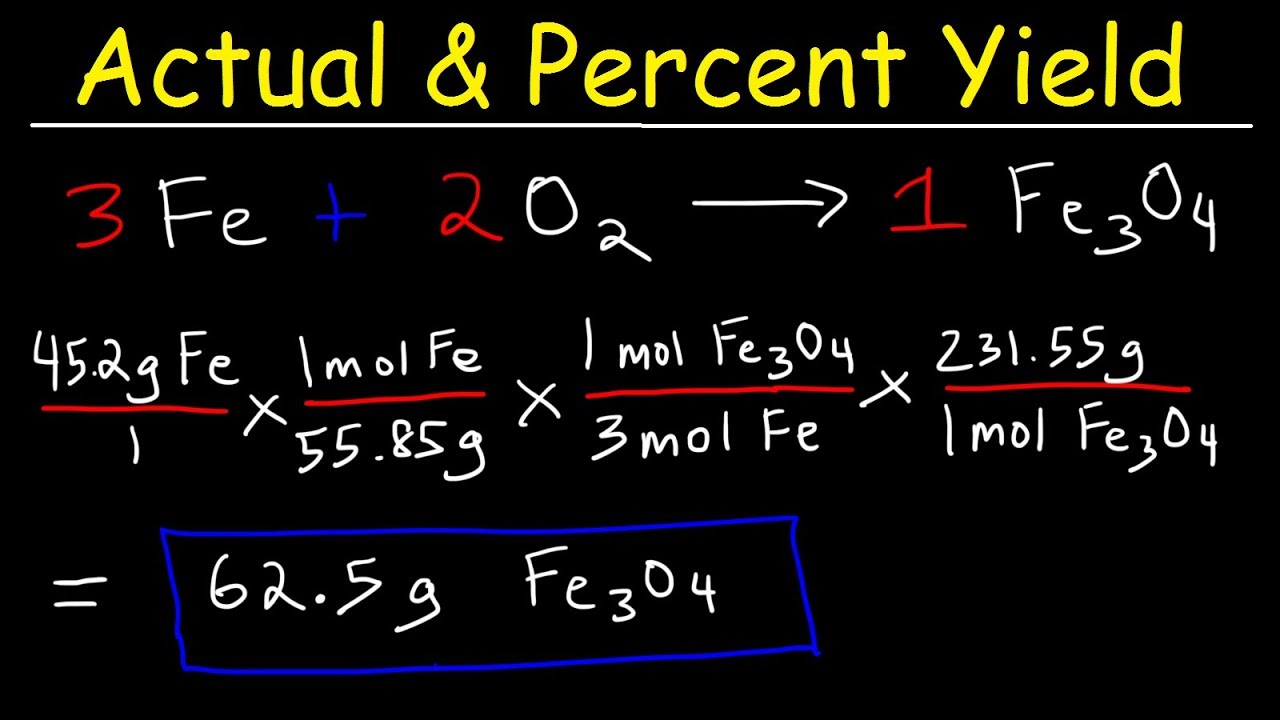
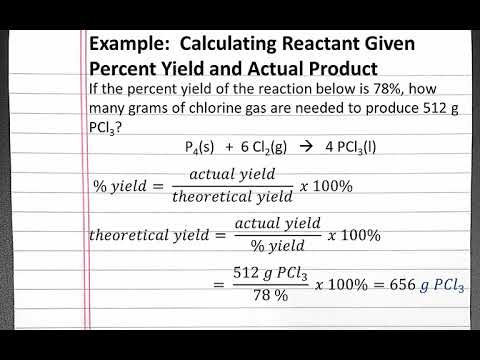
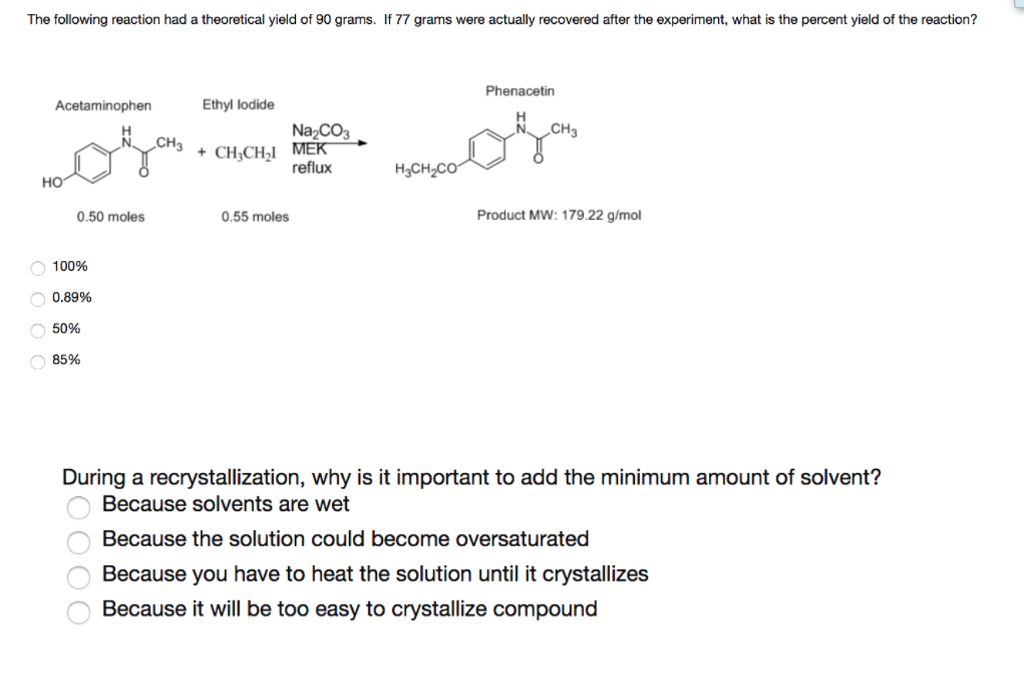
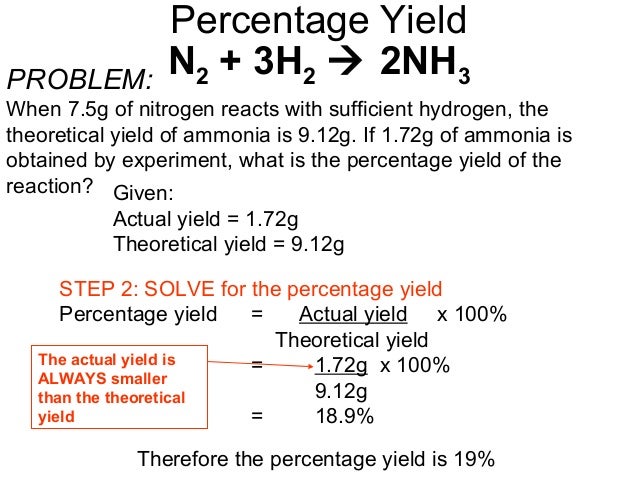
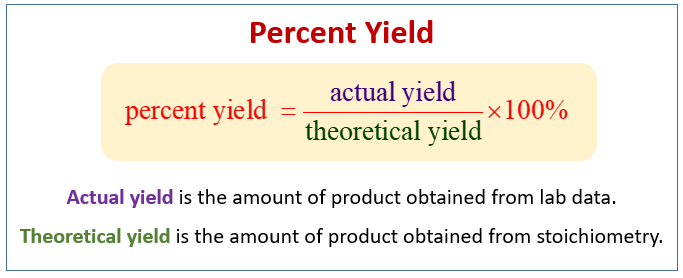
1291 percent yield actual yield theoretical yield 100. Calculating the percent yield of alum. The actual yield is the amount of product that is actually formed when the reaction is carried out in the laboratory.
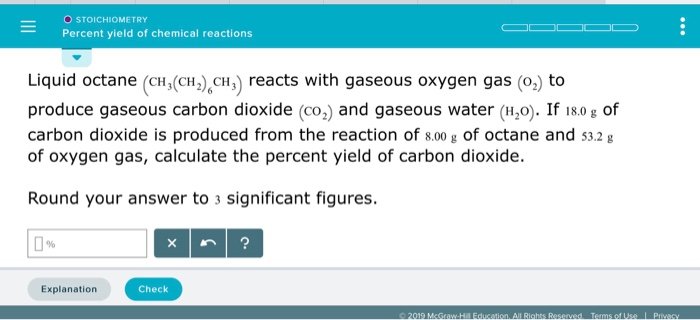
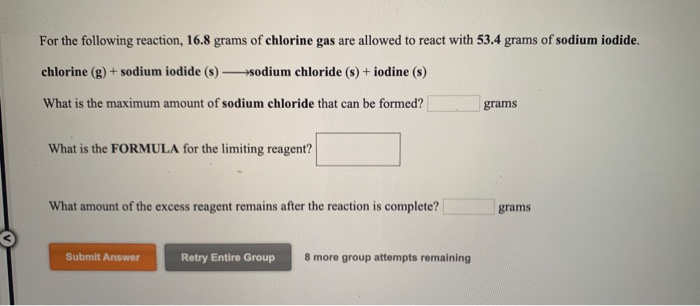
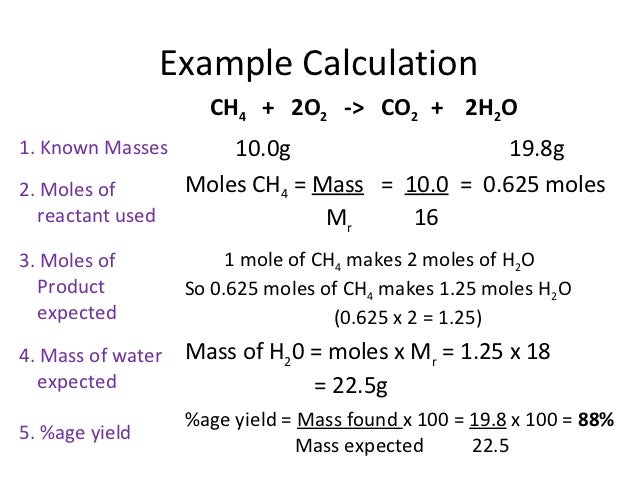
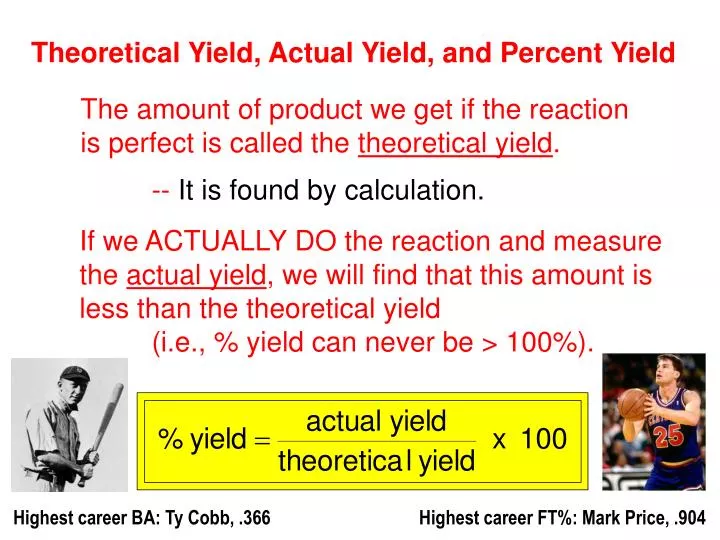
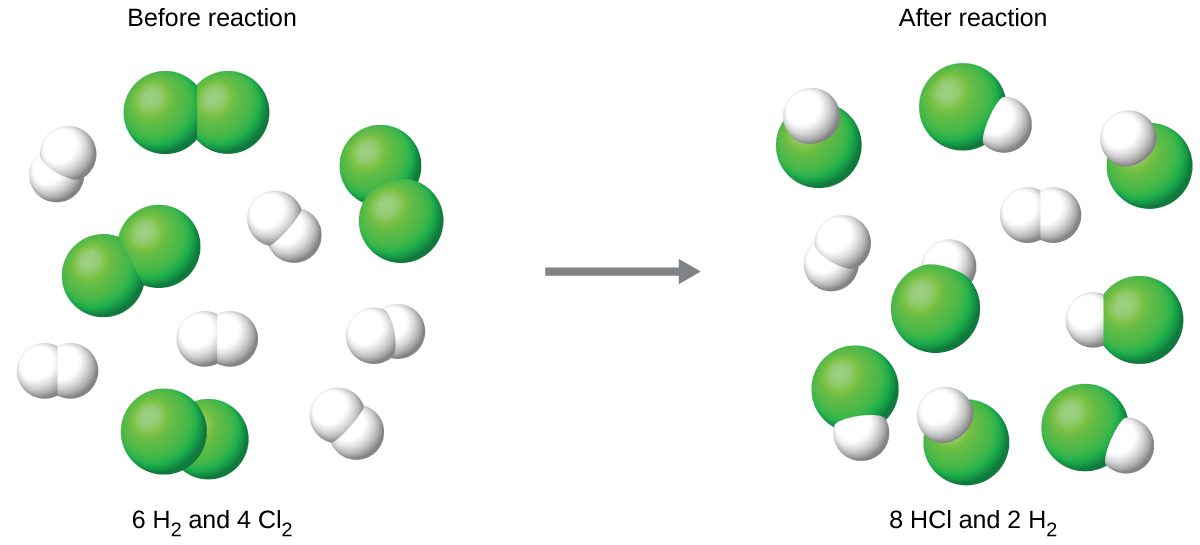
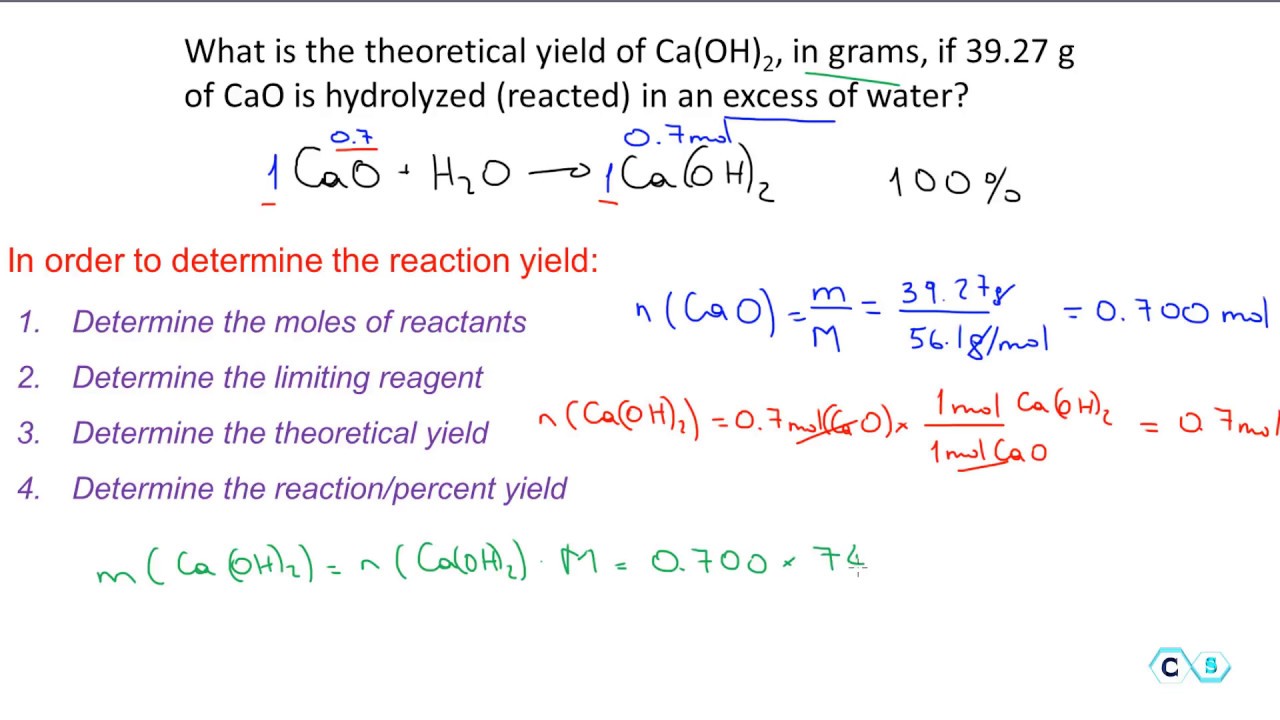
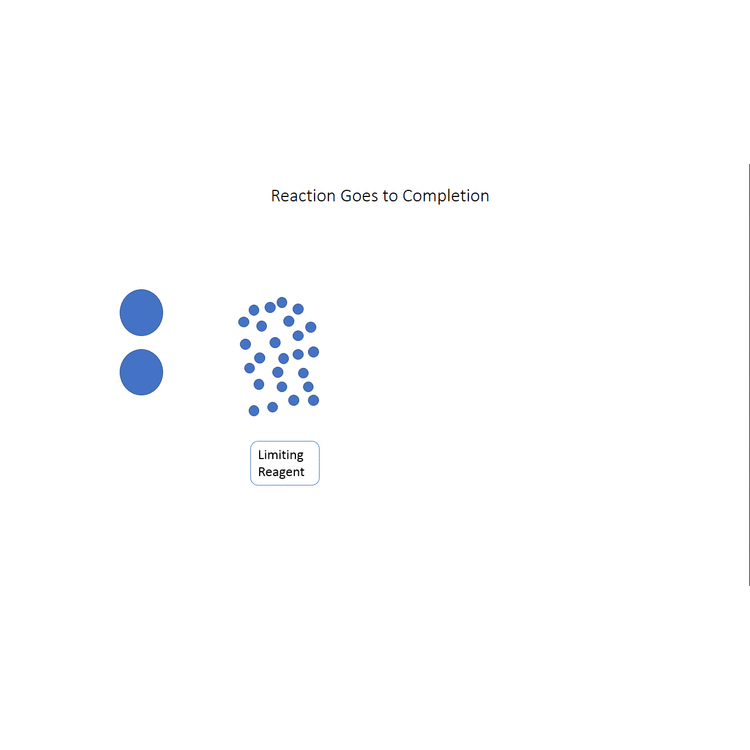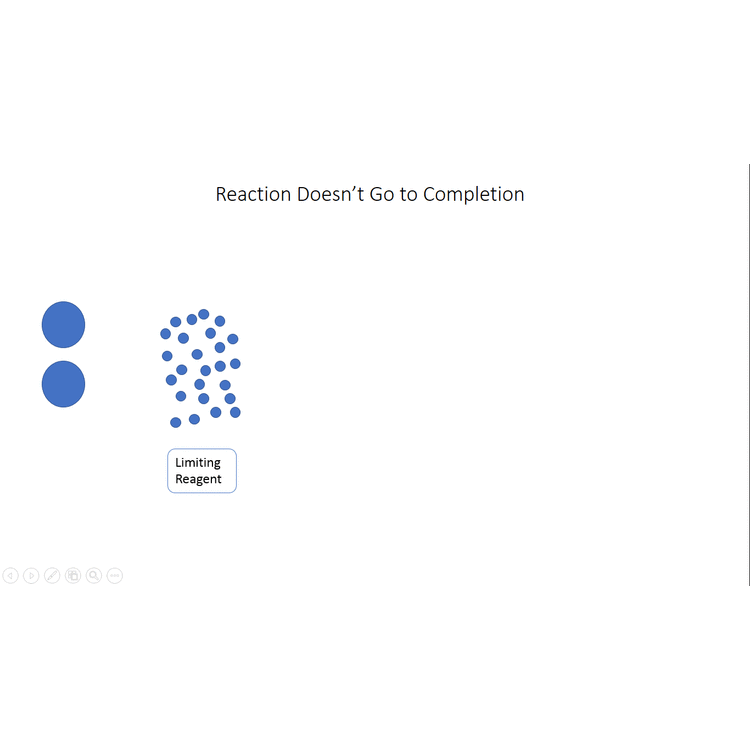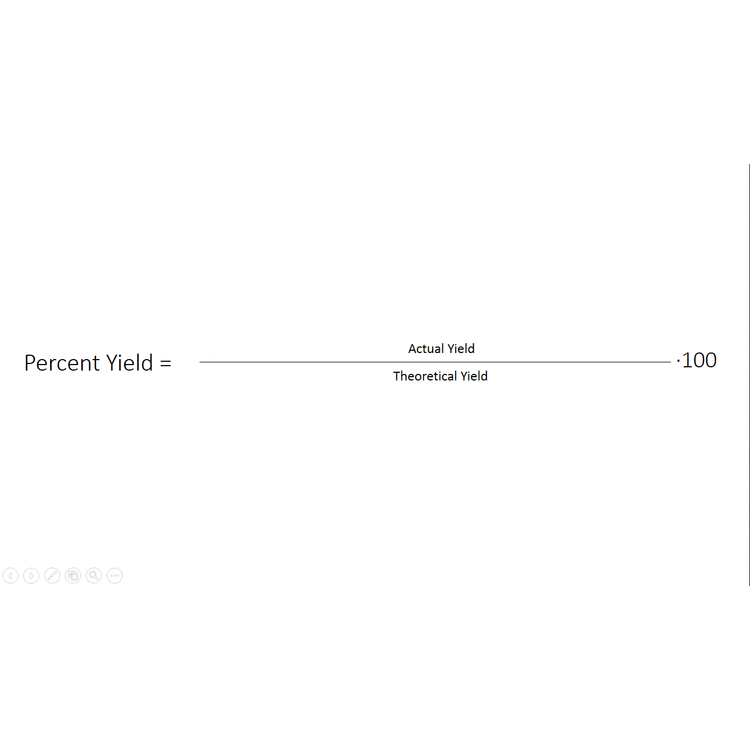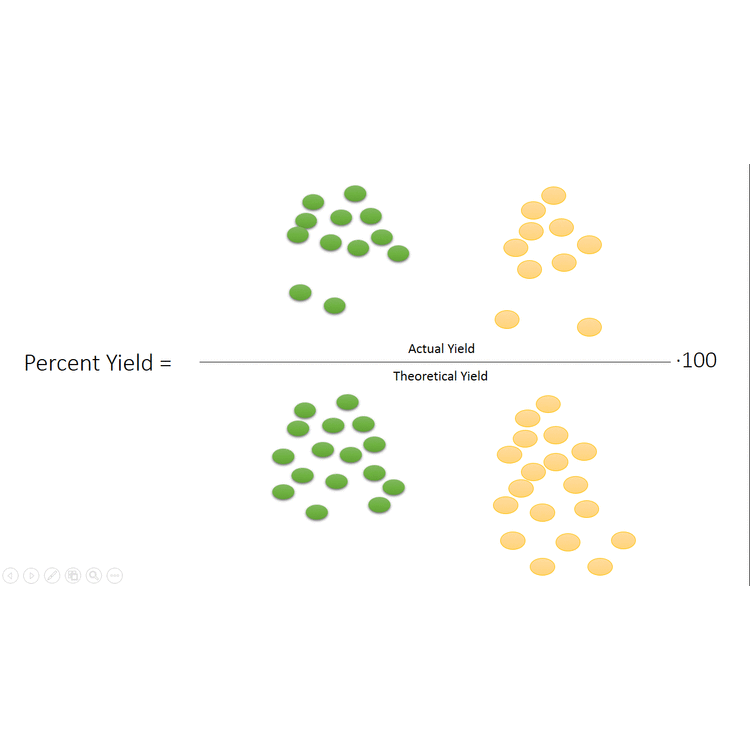
Start with a balanced chemical equation. To determine the percent yield of a product in a chemical reaction we need to know the amount of all reactants used the amount of the product formed and the balanced chemical reaction. This is known as the theoretical yieldthis is a strategy to use when calculating the theoretical yield of a chemical reaction.
So you find that 8137 is the percent yield. So the percent yield 1212 1305 100 929. Lets walk through the steps now.
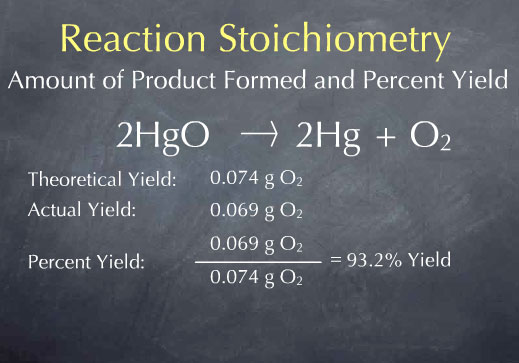
Percent yield theoretical yield and actual yield. How to calculate percent yield in chemistry. Calculating percentage yield the percentage yield is calculated using this equation.
Before performing chemical reactions it is helpful to know how much product will be produced with given quantities of reactants. The percent yield is the ratio of the actual yield to the theoretical yield expressed as a percentage. About the book author.
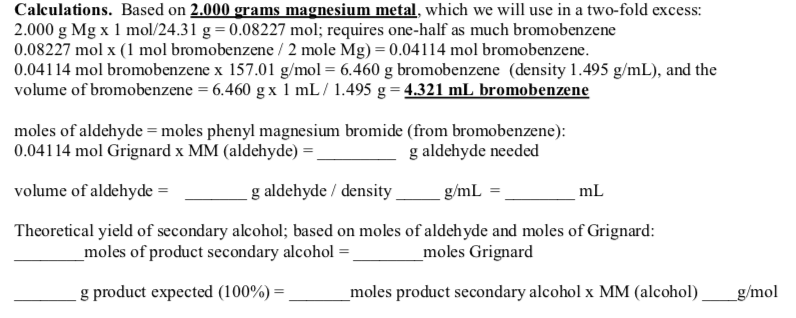
Determine the theoretical yield in gramsnow that we have the quantity of cemath processing error in moles. How to calculate the percent yield of a chemical reaction. A chemical equation describes the reactants on the left side reacting to form products on the right.
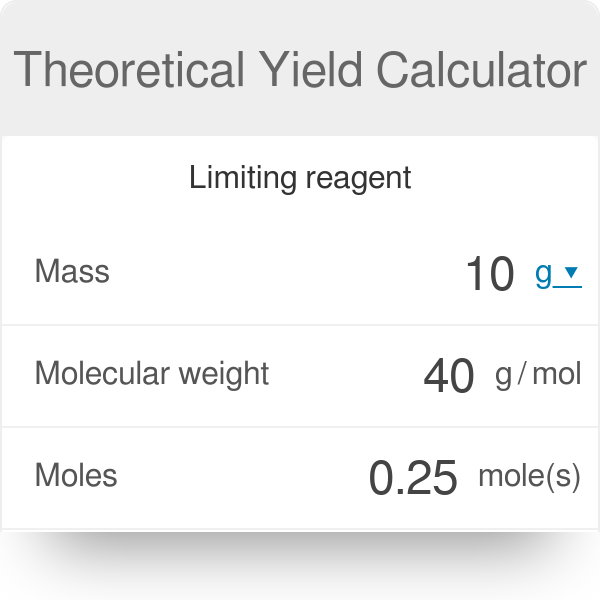
This percent yield calculator is intended to help navigate between three key metrics.

How To Calculate Percent Yield Definition Formula Example Video Lesson Transcript Study Com
study.com